Search Thermo Fisher Scientific
Product Details
MA1-2012
Species Reactivity
Published species
Host/Isotype
Class
Type
Clone
Immunogen
Conjugate
Form
Concentration
Purification
Storage buffer
Contains
Storage conditions
Shipping conditions
RRID
Product Specific Information
MA1-2012 detects the neurofilament, heavy chain in human and rat samples.
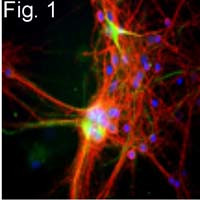
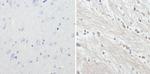
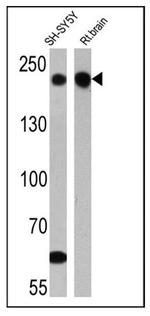
MA1-2012 has been successfully used in Western blot, immunohistochemistry and immunofluorecence procedures. By Western blot this antibody detects a ~200 kDa protein representing the neurofilament, heavy chain in rat brain microsome. In immunohistochemistry procedures MA1-2012 recognizes the neurofilament, heavy chain in rat hippocampal neurons.
The MA1-2012 immunogen is a synthetic peptide corresponding to 226 amino acids of the C-terminus of neurofilament, heavy chain.
Target Information
Involved in the maintenance of neuronal caliber, neurofilaments are the intermediate filament proteins found specifically in neurons, and are composed predominantly of three major proteins called NF-L, NF-M and NF-H. Like most other intermediate filament proteins (IFPs), the expression of the different neuronal IFPs is both tissue-specific and developmentally regulated. NF-H has an important role in mature axons and is involved in amyotrophic lateral sclerosis (ALS).
For Research Use Only. Not for use in diagnostic procedures. Not for resale without express authorization.
Bioinformatics
Protein Aliases: 200 kDa neurofilament protein; NEF3; neurofilament; Neurofilament heavy polypeptide; Neurofilament triplet H protein; neurofilament, heavy polypeptide 200kDa; NF 200 kD; NF-H; NHC
Gene Aliases: CMT2CC; KIAA0845; NEFH; NFH
UniProt ID: (Human) P12036
Entrez Gene ID: (Human) 4744, (Rat) 24587

Performance Guarantee
If an Invitrogen™ antibody doesn't perform as described on our website or datasheet,we'll replace the product at no cost to you, or provide you with a credit for a future purchase.*
Learn more
We're here to help
Get expert recommendations for common problems or connect directly with an on staff expert for technical assistance related to applications, equipment and general product use.
Contact tech support