 Dr. Castle Funatake, Ph.D.
Dr. Castle Funatake, Ph.D.
Castle, a Senior Manager of R&D at Thermo Fisher Scientific, leads a team of R&D scientists responsible for the development of new dyes and novel assays for flow cytometry. Most recently, her team has been involved with the development of the Invitrogen™ eBioscience™ Super Bright antibody conjugates. She speaks to us about the Super Bright dyes and antibody conjugates and shares insights into the performance and tips for use of these antibody conjugates in multiplex flow cytometry applications.
What are the Super Bright antibody conjugates for flow cytometry?
The eBioscience Super Bright antibody conjugates for flow cytometry are our newest, violet laser-excitable antibody conjugates available exclusively from Thermo Fisher Scientific. They are optimized for flow cytometry. Currently available in five unique formats, Super Bright 436, Super Bright 600, Super Bright 645, Super Bright 702, and Super Bright 780, for both human and mouse, these antibody conjugates allow for additional channels off the violet laser while providing better discrimination of dim markers on cell populations. Isotype controls are also available for each of the Super Bright conjugates.

Figure 1. Emission spectra of Super Bright 436, Super Bright 600, Super Bright 645, Super Bright 702, and Super Bright 780 polymer dyes. The black bar indicates the excitation wavelength of the violet laser (405 nm). Use the Fluorescence SpectraViewer, to select these reagents and view the excitation and emission spectra to help design your flow cytometry panels.
Can you tell me more about these reagents for flow cytometry?
The five unique Super Bright dyes are polymer-based, fluorescent dyes that are excited with the violet laser, which is the 405 nanometer laser line. The dyes can be unmodified, which is the Super Bright 436, or they can be modified and made into tandem dyes, which is what the Super Bright 600, 645, 702, and 780 are. As a tandem dye, the unmodified polymer part acts as the donor dye and transfers the energy to the acceptor dye through fluorescence resonance energy transfer (FRET).
The Super Bright antibody conjugates, the dyes themselves, are named for their peak emission wavelengths. The Super Bright 436 has a peak emission of 436 nanometers. The Super Bright 600 is at 600 and so on. They are optimized specifically for use in flow cytometry, and they provide better discrimination of dim populations.

How do the Super Bright polymer dyes work?
The polymer dyes, in a very oversimplified way, are a series of simple organic dyes strung together, but unlike a traditional organic dye, which can self-quench and become dimmer as you increase the number of dyes associated with an antibody, the monomeric units of the polymer dye actually work together to increase the extinction, which is basically just a fancy word for how much light (how many photons) of light a single dye can absorb. The more light that is absorbed, the more light that can be emitted. This is what results in the brightness of the polymer dyes.
The Super Bright line of dyes consist of both the unmodified dye, the Super Bright 436, as well as a series of 4 tandem dyes. You can kind of think of this similarly to PE and the PE tandem. The Super Bright 436 and PE are that unmodified version. They absorb light at their respective wavelengths and then have their own unique emission spectra.
When you make a tandem of the Super Bright with an acceptor dye, this is similar to a PE tandem with eFluor 610 or cyanine5. As a tandem, the Super Bright 436 or PE acts as the donor. So they are the ones absorbing the light from the laser line and then transferring that energy via FRET to the acceptor dyes. The acceptor dyes give the tandems unique emission spectra that is distinguishable from the original unmodified version of the dye, as well as other tandems made with different acceptor dyes. Then these can be conjugated to antibodies for use in flow cytometry, just like other dyes have been.
As with any fluorochrome-conjugated antibody, they should really be titrated for the optimal performance on your cells of interest. They’re unique in that they’re a polymer-based dye and that they’re excited specifically by the violet laser, but in other ways, once they’re conjugated, they behave very similar to other fluorochrome-conjugated antibodies.
What difference will I see in data when I use Super Bright antibody conjugates in flow cytometry?
Better resolution of cell populations. For example, below I’m showing some human peripheral blood cells that were stained with an antibody, the CD20 PE shown on the X axis. On the Y axis, we’re looking at CD11c conjugated to Super Bright 702 on the left, and you can see that the cells in the upper left quadrant are much better resolved from the cells in the lower left quadrant when we compare that to the CD11c APC staining shown on the right plot.

What are the characteristics of each Super Bright polymer dye conjugate?
Super Bright 436
This is the unmodified polymer dye, and it is an alternative to Brilliant Violet™ 421. It has a peak emission of 436 nanometers, and it can be detected using a 450/50 bandpass filter or equivalent in flow cytometry. It does show superior performance when we compare it to eFluor 450.
In the data below, we are looking at mouse bone marrow cells that have been stained with Sca-1 APC shown on the X axis, and then CD117 shown on the Y axis. The first plot on the left, we are looking at the staining of the eFluor 450. The middle plot is the Super Bright 436. On the far right is the Brilliant Violet™ 421. You can see that the Super Bright 436 provides much brighter staining than the eFluor 450 and is comparable to the Brilliant Violet™ 421.

Super Bright 600
Super Bright 600 is an alternative to Brilliant Violet™ 605 and it is a tandem of Super Bright 436 and an acceptor dye with a 600 nm peak emission. It can be detected using a 610/20 bandpass filter or equivalent in flow cytometry. The data shown here are mouse splenocytes stained with CD8 antibody. The Brilliant Violet™ is shown in gray, and the Super Bright 600 is shown in red. You can see that we have very bright and similar performance.

Super Bright 645
The Super Bright 645 is also a tandem, and it is an alternative to Brilliant Violet™ 650. It can be detected using a 660/20 bandpass filter in flow cytometry. One of the nice features about the Super Bright 645 is that it has reduced compensation out of some of the other violet laser channels, and in some cases (due to dependency on instrument and PMT), reduced compensation is also seen out of the 450 channel as compared to the Brilliant Violet™ 650. The reduced compensation makes it easier to incorporate the Super Bright 645 into multi-color panels that include other violet dyes.

In the data below, mouse splenocytes are stained with CD8 on the left or human peripheral blood cells are stained with CD8 on the right. Again, the Brilliant Violet™ 650 is shown in gray, and our Super Bright 645 is shown in red. You can see that the two reagents have very similar performance.

Super Bright 702
Super Bright 702 is a tandem of Super Bright 436 and an acceptor dye, and it’s an alternative to the Brilliant Violet™ 711. It can be detected using a 710/50 bandpass filter in flow cytometry. Similar to the Super Bright 645, Super Bright 702 has reduced compensation out of the PerCP-eFluor 710 channel off of the blue laser and also less compensation out of the Brilliant Violet™ 786 channel off the violet laser. Again, this makes using the Super Bright in your multi-color panels quite a lot easier.

In the data shown below, we’re looking at mouse splenocytes stained with CD4 on the left and human peripheral blood cells stained with CD19 on the right. The Brilliant Violet™ 711 is shown in gray. The Super Bright 702 is shown in red. Again, we have very comparable, nice, bright staining.

Super Bright 780
Super Bright 780 is an alternative to Brilliant Violet™ 786 and it is a tandem of Super Bright 436 and an acceptor dye with a 780 nm peak emission. It can be detected using a 780/60 bandpass filter or equivalent in flow cytometry and has less spillover into other violet laser channels.
Mouse splenocytes were stained with anti-CD4 conjugated to Super Bright 780 (top row) or with a Brilliant Violet 786 conjugate (bottom row). You can see that Brilliant Violet 786 is under-compensated relative to Super Bright 780. Actual compensation values for both dyes shown in the table below.


How stable are the Super Bright dyes to fixation buffer or when stored in formaldehyde fixation solution overnight?
The Super Bright dyes themselves are quite stable. In the flow cytometry plots below, on the top left is mouse splenocytes stained with CD8 Super Bright 645. On the bottom left, we’re looking at human peripheral blood cells stained with CD3 Super Bright 702. These cells were analyzed either fresh, which is shown in the red histogram, or the cells were fixed with the IC Fixation buffer and stored for 30 minutes shown in blue or analyzed after one day shown in orange or analyzed after three days shown in green. What you can see is that there is really very minimal loss in signal intensity even after three days of storage in fixation buffer.

In addition, although it’s not shown here, we don’t see a substantial change in the compensation values. Like the other tandem dyes, we recommend that if you can’t analyze your samples on the same day, it’s actually best to store them in a high-quality formaldehyde fixation solution because this preserves not only the brightness but also the FRET, which preserves any compensation values – if you need to analyze your samples several days after staining.
In addition to being stable in fixation buffers, we’ve also looked at the stability of samples that were stained and then left on the lab bench for four hours or overnight (above figure, on the right). On the right-hand side, the fresh cells are shown in red. Samples left for four hours are shown in orange, and samples left on the bench overnight are shown in blue. At the top right, we’re looking at human peripheral blood cells that were stained with CD19 Super Bright 436. In the above, bottom right figure, we are looking at mouse splenocytes stained with CD4 Super Bright 600. Again, you can see that there is really very little loss of signal intensity. Like the fixation studies, we don’t see a substantial difference in the compensation values. Once your cells are stained, they’re quite stable. These were stored either on the bench or the fresh samples were collected fresh. If you forget about your samples or step away from the bench for a couple of hours, your samples are still okay to run without any change in performance. As a reminder, because many of Super Bright antibody conjugates are tandems, the reagent vials should be protected from light in order to reduce unnecessary exposure of the reagent to light.
Any compatibility issues?
The Super Bright dyes are fully compatible with standard flow cytometry protocols. We’ve evaluated their performance following IC Fixation and Permeabilization Buffer , and also, following the use of the FoxP3/Transcription Factor Staining Buffer Set, as well as protocols that utilize the IC Fixation and methanol permeabilization reagents. We see very little change in performance.
The Super Bright dyes are also compatible with whole blood and lysed-whole blood staining protocols. This includes protocols where you would stain the whole blood and then follow that with a lysis buffer, whether that’s a standard RBC (red blood cell) Lysis or a Fix and Lyse protocol, or whether you pre-lyse the red blood cells and then do your staining.
Additionally, the Super Bright dyes can be used with UltraComp eBeads. In fact, this is the preferred method for setting compensation when using Super Bright dyes and large panels.
Anything I should know about before I begin working with Super Bright conjugates?
Antibody conjugates of polymer dyes, when used in combination, can display some nonspecific interactions. To eliminate this phenomenon, we developed the Super Bright Staining Buffer. It’s not so much a staining buffer, rather, it’s a reagent that you actually add to your cells or to your cocktails before you add any polymer-based dyes, including the Super Bright. It is formulated at 5 microliters per test. This makes it much easier to use when you’re putting together large multi-color panels for flow cytometry because it will not affect the dilution of the reagents. The 5 microliter formulation is also great to use when space is an issue, for instance, when staining in 96-well plates. Use of the Super Bright Staining Buffer is recommended when you’re using two or more Super Bright conjugates in the same panel, or if you are combining the Super Bright conjugates with Brilliant Violet™ reagents.
To see how the flow cytometry data changes with and without the addition of the buffer, see the figure below. Shown are human peripheral blood cells that were stained with CD8 Super Bright 600 on the Y axis and either CD4 Super Bright 436 shown on the left side or CD4 Brilliant Violet™ 421 shown on the right. In the top row, Plots A and C, we’re showing what the staining looks like if we just do staining in our normal flow cytometry staining buffer. You can see that the cells that are CD4+ in the lower right quadrant are starting to kind of creep up and look undercompensated, however that’s not what’s going on. We know that the compensation has been set correctly. We know that this is a phenomenon of the two polymer dyes interacting with each other.

If we add Super Bright Staining Buffer to the cells before we add our Super Bright conjugated antibodies, this data is shown in the lower plots, B and D. You can see that we eliminate this nonspecific interaction. This data shows that the Super Bright Staining Buffer works both on Super Bright conjugates when it’s an all Super Bright panel, as well as the combination of a Brilliant Violet™ antibody conjugates and Super Bright antibody conjugates.
In another example below, we’re again looking at human peripheral blood that was stained with CD4 Brilliant Violet™ 421 on the X axis, and CD8, conjugated to either Super Bright 600 or Brilliant Violet™ 605 on the Y axis. In the top row, you can see, again, if we do the staining in the presence of flow cytometry staining buffer-only, we see that there are some non-specific interaction between the Brilliant Violet™ 421 and the Super Bright 600, as well as the Brilliant Violet™ 605 shown in the far right plot.
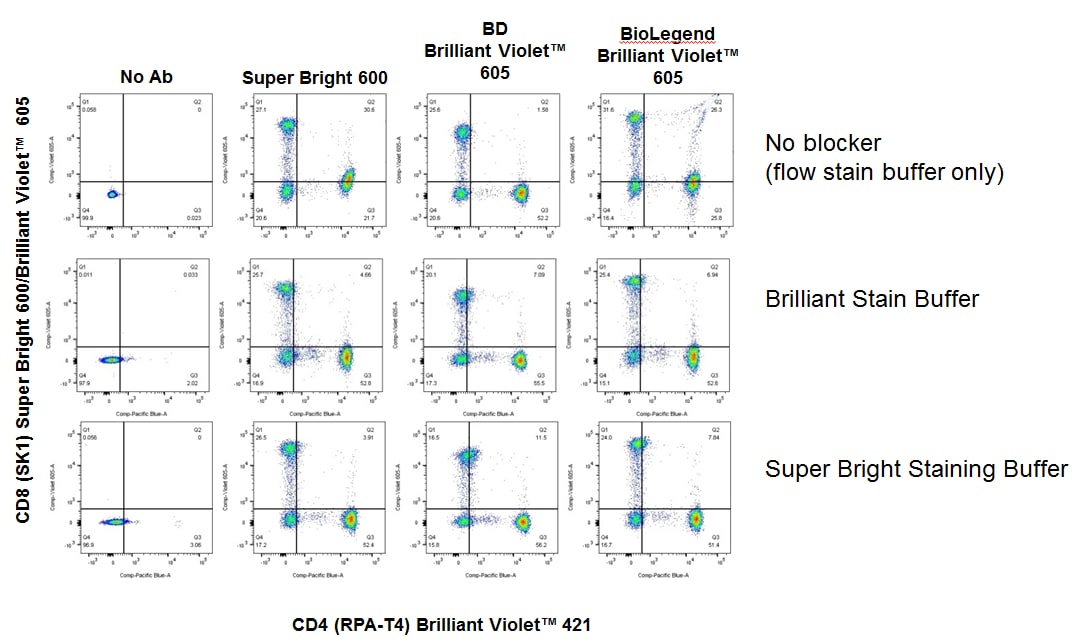
If we add Brilliant Stain Buffer before we do the staining, which is shown in this middle row, you can see that we eliminate that nonspecific interaction and likewise, if we use our Super Bright Staining Buffer shown in the bottom row, this also eliminates the nonspecific interaction between the dyes. Whether the panel is all Brilliant Violet™ conjugates, all Super Bright conjugates or some combination of the two, the Brilliant Stain Buffer and the Super Bright Staining Buffer can eliminate these nonspecific interactions.
Are there any differences between the Super Bright Staining Buffer as compared to the Brilliant Violet™ Staining Buffer?
Yes. The two buffers are similar in that they both prevent any undesired, nonspecific dye-dye interactions that can happen when using multiple polymer-based dyes. The Super Bright Staining Buffer is compatible with Brilliant Stain Buffer. The one nice thing about it is that it’s formulated at 5 microliters per test in contrast to the 10 microliters per test of the Brilliant Stain Buffer. This makes it easier to use with the multi-color panel because it has a smaller effect on the overall dilution of your reagents when you’re adding them to the cells. Also, it requires less volume if space in the tube (or well) is an issue.
How do the Super Bright conjugates perform in flow cytometry panels to study different cell subsets?
In the example panel below, it was designed to identify and characterize ILC2 in human peripheral blood. This is a 10-color panel that highlights the utility of using Super Bright antibody conjugates to identify a rare event like the CD127+ events for ILCs and shows the expression of dim markers (such as KLRG1 or CD117) or markers that are expressed with a broad expression pattern like CD45.
We started with a lineage marker and gated on the negatives (below, top left plot). Then we looked at expression of CD127 versus CD45 (top middle plot). Both of these are Super Bright antibody conjugates, and you can see in this middle plot of the top row that we can identify the CD127+ events. These are the total ILCs, and then the CD127- population is everything that is not an ILC cell type.
Then in the next plot over, in the top right, we’re looking at CD127 versus CD7, and you can see that the ILCs shown in red have a much higher level of expression of CD7; whereas the non-ILCs have both positive and negative cells for CD7.

We can further characterize the ILCs based on expression of CD294 (also known as CRTH2) and CD161 (above, middle row). If we gate on the cells that are double positive (purple box, middle row, left plot), those are the ILC2s (middle row, shown in purple). We can compare the expression of additional markers on those ILC2s against everything that’s not an ILC. You can see that we can see some expression of KLRG1, which is conjugated to Super Bright 702, as well as CD196 (CCR6) and CD117 (c-Kit). We can also look at the cells that although they are ILCs based on their CD127 expression, they are negatives for CRTH2 and then we can characterize their expression of CD117 and NKp46, which is shown in the two-parameter plot with the blue contours in the bottom row.
Can the Super Bright conjugates be used to prepare antibody cocktails for flow cytometry ahead of time?
Yes. The Super Bright Staining Buffer can be used in combination with polymer dyes to prepare antibody cocktails. The Super Bright Staining Buffer should be added before the addition of any Super Bright or Brilliant Violet™ conjugated antibodies, but it can be used either before or after other traditional fluorochrome dyes. These cocktails can then be stored overnight before use. We don’t recommend storing them for longer than 24 hours because some of that nonspecific dye-dye interaction will start to happen.
In addition, we know that we can also incorporate viability dyes. I showed one example of the fixable viability dye eFluor 780, but other DNA intercalating dyes like propidium iodide or 7-AAD can also be used following just their standard protocols. They’re definitely compatible with multi-color cocktails and panels.
Any closing remarks about the Super Bright antibody conjugates?
To summarize, the Super Bright antibody conjugates are currently available in 5 different formats of antibody conjugates (Super Bright 436, Super Bright 600, Super Bright 645, Super Bright 702, and Super Bright 780) across a wide variety of human and mouse antibodies; isotype controls are also available. The portfolio will continue to expand going forward. You can search for your Super Bright antibody conjugate at thermofisher.com/superbright
Brilliant Violet is a trademark of Becton, Dickinson and Company.
For Research Use Only. Not for use in diagnostic procedures. Not for resale. Super Bright Polymer Dyes are sold under license from Becton, Dickinson and Company.
Leave a Reply