Cell therapy manufacturers face several challenges in developing the appropriate tests to analyze and accurately characterize their biological products. This blogpost will discuss these challenges as well as the current characterization strategies and tools that manufacturers use to overcome existing hurdles. For more information, check out the accompanying chapter on characterization and analysis (chapter 8) in our Cell Therapy Handbook.
Defining product specifications
As defined in ICH Q6B, the characterization of a biological product includes, “determination of physiochemical properties, biological activity, immunochemical properties, purity and impurities”. By definition, a specification consists of the assay or test, the protocol for performing it, and the numerical limits, ranges, or other observations that define a product’s acceptance criteria. These acceptance criteria can be divided into several categories—Identity, Purity, Potency, Safety, and Other.
Table 1. Categories of acceptance criteria for new cell therapies.
| Category | Definition |
| Identity
21CFR610.14 |
· Assays that identify the product for proper labeling and will distinguish the product from other products manufactured in the same facility.
· Examples include cell surface or intracellular markers, gene expression, secreted molecules, and peptide sequences. |
| Purity
21CFR600.3 |
· Assays covering residual testing for materials used during the manufacturing process (e.g., isolation or activation beads, digestion enzymes, or genetic engineering reagents).
· Can refer to contaminating cell types that may have an adverse effect on final product safety and efficacy. |
| Potency
21CFR600.3 |
· A measure of biological activity that demonstrates the capacity of a cell therapy product to affect a given result (21CFR600.3).
· A matrix of assays is recommended because of difficulty selecting a single assay that assesses product quality and consistency while predicting clinical efficacy. |
| Safety | · Assays that test mainly for adventitious agents (e.g., sterility, mycoplasma, or endotoxin levels).
· Can also include other areas of concern (e.g., immunogenicity or tumorigenicity). |
| Other | · Includes tests for appearance, dose, viability, and cell counts. |
Quality by design approach
As defined by the ICH, Quality by Design (QbD) is “a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management.”
See below for a general decision framework scheme that cell therapy developers must make to define and control the quality of a cell therapy.
- The first step is to formulate a hypothesis on a product’s Mechanism of Action (MOA) — the specific action(s) a cell product will produce to achieve a desired therapeutic effect.
- The next step is to define a Target Product Profile (TPP) — the characteristics that assure quality, safety, and efficacy.
- Third, the developer must establish which physical, chemical, biological, or microbiological properties must be controlled within an appropriate limit, range, or distribution to achieve the desired clinical outcome; these are known as Critical Quality Attributes (CQAs).
- Lastly, the Critical Process Parameters (CPPs) that affect CQAs are identified, and their effects on those attributes are quantified in a design space. A control strategy is developed to ensure that CPPs remain within the “normal operating range” that ensures manufacture of high-quality product.
Regulatory perspective and development stages
Given the complexity of cell-based products and the diversity of indications they are meant to treat, no single framework exists to govern how these therapies are evaluated. Instead, each characterization scheme must be tailored to a particular product.
An example of an incremental approach for stage-specific assay development for cell therapy characterization is described below.
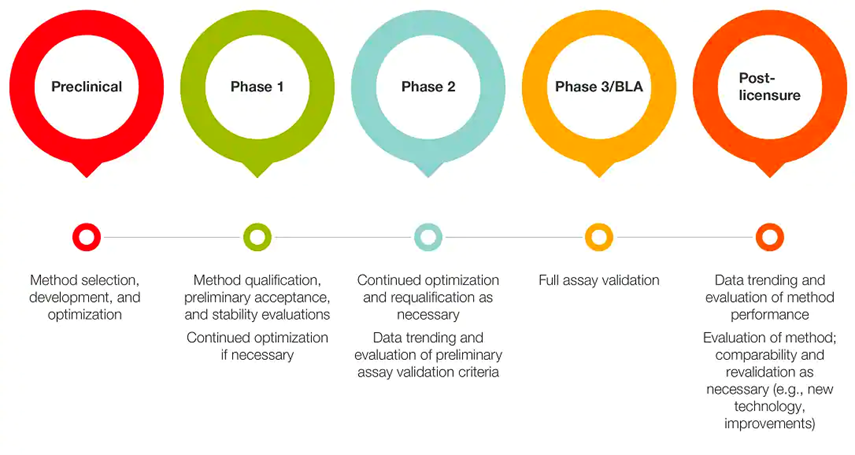
Figure 1. Stage-specific assay development for cell therapy characterization.