 Genome editing is not a new concept to the scientific community and has been around for decades. However, directing precise sequence changes at desired sites has remained a difficult and tedious challenge for researchers. Limited successes have been achieved with oligonucleotides, small molecules, or self-splicing introns, but the development of site-directed zinc finger nucleases (ZFNs) and TAL effector nucleases (TALENs) has facilitated sequence-specific manipulations. Nevertheless, difficulties of protein design, synthesis, and testing have slowed adoption of these engineered nucleases for routine use in genome editing experiments. The most recent gene editing technology, the CRISPR-Cas9 system, largely overcomes these difficulties, making it an attractive method for genome editing. It is critical that we adopt a workflow and use technology that can make genome editing efficient by accurately identifying on- and off-target mutations, producing a gene-edited organism that may have fewer or no off-targeted gene side effects1, 2. Today, with the aid of gene editing tools, researchers can quickly generate model organisms that can be used to study human diseases, test efficacy of various drugs, and even create genetically modified organisms during times of an epidemic, as has been done for the Aedes aegypti mosquito, a vector for the Zika virus1, 2, 3.
Genome editing is not a new concept to the scientific community and has been around for decades. However, directing precise sequence changes at desired sites has remained a difficult and tedious challenge for researchers. Limited successes have been achieved with oligonucleotides, small molecules, or self-splicing introns, but the development of site-directed zinc finger nucleases (ZFNs) and TAL effector nucleases (TALENs) has facilitated sequence-specific manipulations. Nevertheless, difficulties of protein design, synthesis, and testing have slowed adoption of these engineered nucleases for routine use in genome editing experiments. The most recent gene editing technology, the CRISPR-Cas9 system, largely overcomes these difficulties, making it an attractive method for genome editing. It is critical that we adopt a workflow and use technology that can make genome editing efficient by accurately identifying on- and off-target mutations, producing a gene-edited organism that may have fewer or no off-targeted gene side effects1, 2. Today, with the aid of gene editing tools, researchers can quickly generate model organisms that can be used to study human diseases, test efficacy of various drugs, and even create genetically modified organisms during times of an epidemic, as has been done for the Aedes aegypti mosquito, a vector for the Zika virus1, 2, 3.
Understanding the CRISPR-Cas9 gene editing system
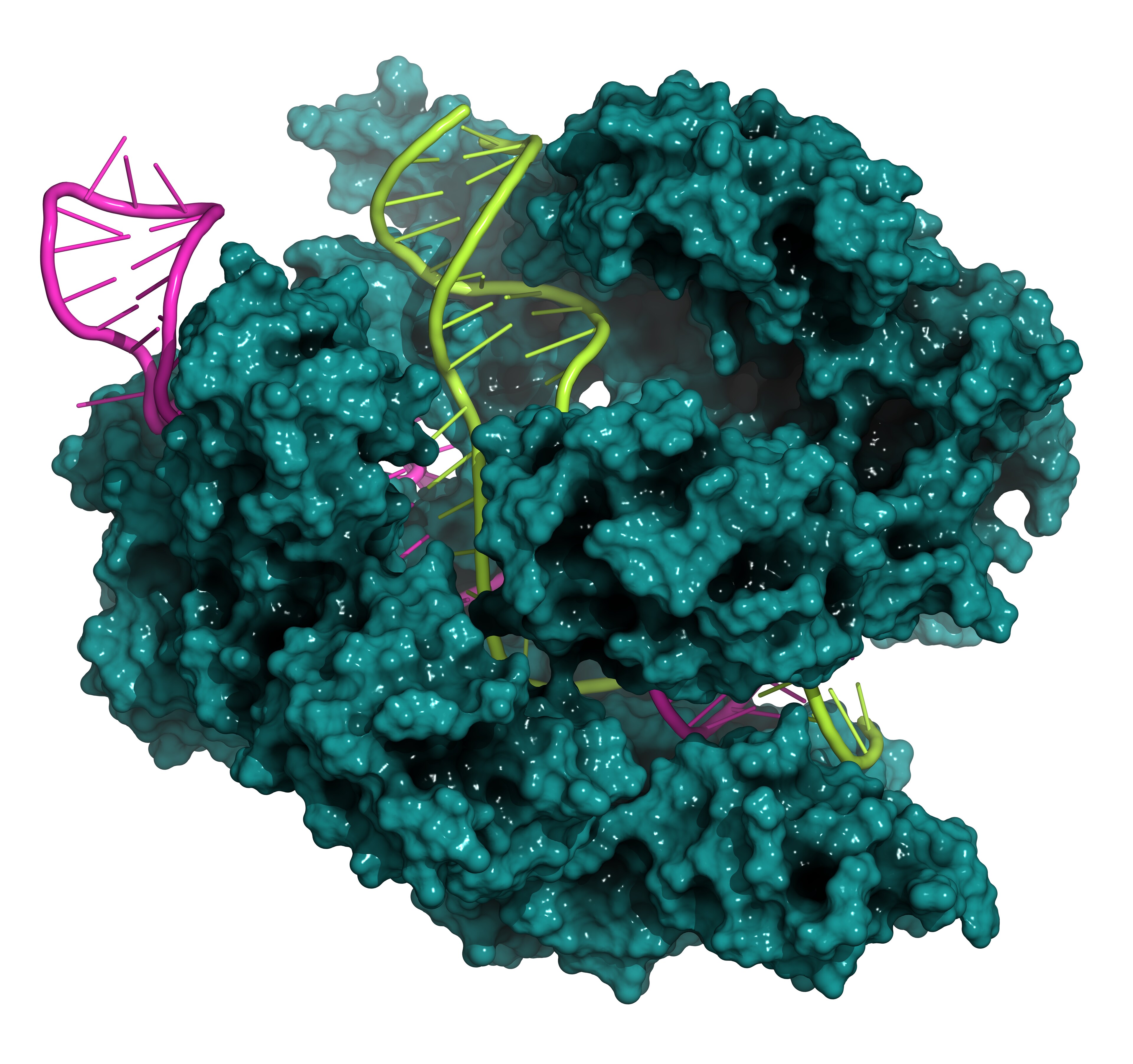
Clustered regularly interspaced short palindromic repeats (CRISPRs) belong to a class of repeated DNA sequences that work together with CRISPR-associated (Cas) genes to protect bacteria and archaea from invading foreign nucleotides, such as those from phages and plasmid DNA1, 2. Upon injection of phage-DNA into the bacteria, the phage-DNA is cut into small pieces and incorporated into the CRISPR locus1, 2. This locus, now containing information about the foreign phage-DNA is transcribed, this CRISPR-RNA (crRNA) along with a second non-coding RNA, trans-activating CRISPR RNA (tracrRNA), then forms a ribonucleoprotein complex with the Cas9 endonuclease and cuts the foreign DNA from this specific phage1, 2. So, a bacteria carrying information about a specific phage-DNA can have immunity towards attack from that phage1, 2. In this way, bacteria and archaea have evolved an adaptive immune response against invading DNA and can protect themselves in the future when attacked by the same phage1, 2.
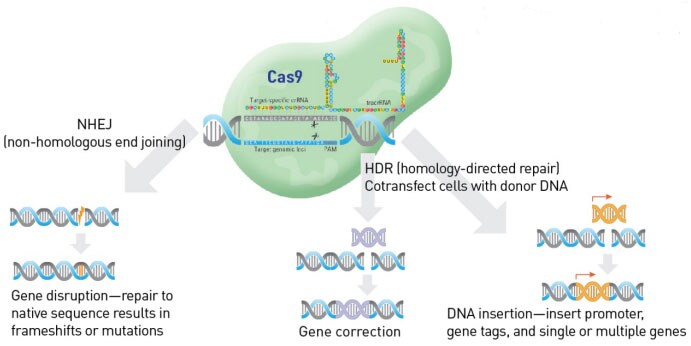 This CRISPR-Cas9 system that provides adaptive immunity against foreign elements in bacteria and archaea, has been modified today to be able to edit genes in various cell types (mammalian, plant, insect, fungi, etc)1, 2. Researchers combined the crRNA and tracrRNA and generated a single guide RNA (sgRNA), which recruits the Cas9 nuclease to specific genomic locations with guidance from the sgRNA. sgRNA then pairs with the complimentary genomic sequences via Watson-Crick pairing and the Cas9 nuclease induces double strand breaks (DSB)1, 2. The DSB can be corrected by an error-prone mechanism called, non-homologous end joining (NHEJ), which causes insertions or deletions1, 2. If a synthetic template is present, the DSB can also be corrected by homology-directed repair (HDR), allowing the introduction of desired base-changes into the genome1, 2.
This CRISPR-Cas9 system that provides adaptive immunity against foreign elements in bacteria and archaea, has been modified today to be able to edit genes in various cell types (mammalian, plant, insect, fungi, etc)1, 2. Researchers combined the crRNA and tracrRNA and generated a single guide RNA (sgRNA), which recruits the Cas9 nuclease to specific genomic locations with guidance from the sgRNA. sgRNA then pairs with the complimentary genomic sequences via Watson-Crick pairing and the Cas9 nuclease induces double strand breaks (DSB)1, 2. The DSB can be corrected by an error-prone mechanism called, non-homologous end joining (NHEJ), which causes insertions or deletions1, 2. If a synthetic template is present, the DSB can also be corrected by homology-directed repair (HDR), allowing the introduction of desired base-changes into the genome1, 2.
The CRISPR-Cas9 system is one of many and there are probably many such tools that have yet to be discovered. Using Sanger sequencing, performed on the Applied Biosystems™ 3130xl genetic analyzer, Ivanov et al were able to discover CRISPRs in a newly discovered Bordetella species4. The Cas9 endonuclease gene in the Bordetella species is 990bp, which is smaller than that of Streptococcus pyogenes Cas9 (SpyCas9) gene4. The CRISPR-SpyCas9 system is the most widely studied and used, the newly discovered smaller Bordetella Cas9, which is equally functional and active as the SpyCas9 might be an attractive alternative for the widely used CRISPR-Cas9 system4.
Improving the CRISPR-Cas9 workflow
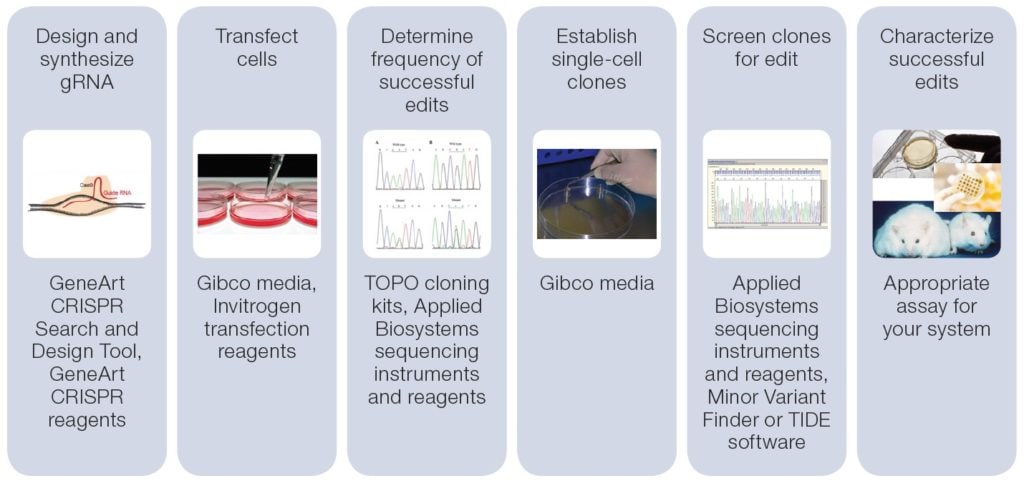
We’ve provided an end-to-end integrated workflow with all the tools necessary for genome editing and downstream analysis. The Invitrogen™ GeneArt™ design tool facilitates the design and ordering of target specific gRNAs for CRISPR-mediated genome editing or TALs for TALEN mediated genome editing. Invitrogen™ transfection reagents offer several options for delivery of genome editing tools into eukaryotic cells. In addition, Invitrogen™ TOPO™ TA cloning vectors and competent cells facilitate the sequence analysis of primary transformants. Gibco™ media is available for growing the primary transformants and secondary cultures following clonal expansion. Finally, Applied Biosystems™ sequencing instruments, reagents and software (Applied Biosystems™ Minor Variant Finder Software or TIDE software) enable the determination of specific genomic editing events.
For accurate and efficient gene editing, it is critical that we confirm the sequences of the guide RNAs and synthetic templates used for DSB repair, to assure efficient targeting. Sanger sequencing can not only help in testing the sequence of the sgRNA, but also in screening cells post-gene editing, to check for on- and off target mutations and to make sure there are no unwanted insertions, deletions or mutations that may possibly lead to frameshift mutations.
Applications of CRISPR-Cas9 gene editing system
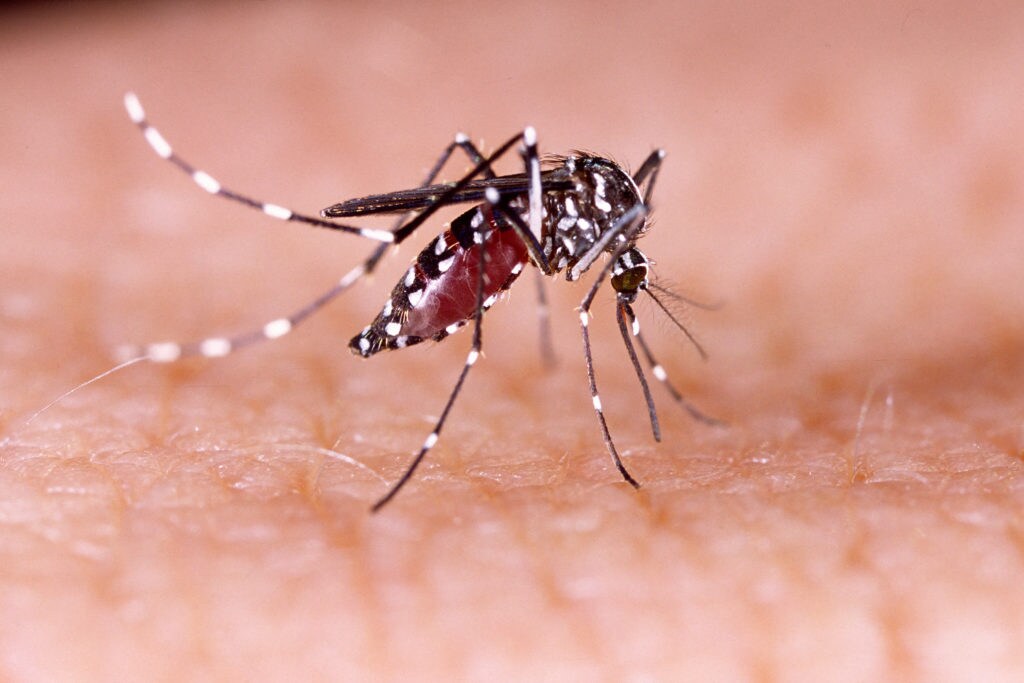 As the Zika epidemic continues, researchers have been trying to figure out a way to reduce the spread and growth of the Aedes aegypti mosquito, which is a vector for Zika and various other viruses3. Dong et al showed for the first time that the CRISPR-Cas9 system could be used to disrupt a gene in the Aedes Aegypti mosquito3. They used the Applied Biosystems™ 3730xl genetic analyzer to sequence the DNA of regions targeted by the CRISPR-Cas9 system, validating their results3. Such research opens up the application of a specific workflow to various organisms, showing that genome editing can be easily used to insert or delete genes in an organism of interest.
As the Zika epidemic continues, researchers have been trying to figure out a way to reduce the spread and growth of the Aedes aegypti mosquito, which is a vector for Zika and various other viruses3. Dong et al showed for the first time that the CRISPR-Cas9 system could be used to disrupt a gene in the Aedes Aegypti mosquito3. They used the Applied Biosystems™ 3730xl genetic analyzer to sequence the DNA of regions targeted by the CRISPR-Cas9 system, validating their results3. Such research opens up the application of a specific workflow to various organisms, showing that genome editing can be easily used to insert or delete genes in an organism of interest.
 Research has shown that when the MSTN gene is knocked out it increases skeletal muscle in mice. Rao et al were interested in generating Myostatin (MSTN)-knockout (KO) pigs using genome editing technology5. Using TALEN genome editing technology, they generated MSTN-KO somatic porcine cells5. The team used Sanger sequencing or Capillary Electrophoresis (CE), performed on the Applied Biosystems™ 3130 genetic analyzer, to determine mutations, small insertions or deletions and off-target mutations5. Cells carrying the correct KO were then used for somatic-cell nuclear transfer (SCNT) to generate MSTN-KO piglets5. Interestingly, these MSTN-KO piglets were higher in body weight and displayed increased muscle mass, compared to wildtype5. Such studies can compliment breeding efforts to help increase pork production.
Research has shown that when the MSTN gene is knocked out it increases skeletal muscle in mice. Rao et al were interested in generating Myostatin (MSTN)-knockout (KO) pigs using genome editing technology5. Using TALEN genome editing technology, they generated MSTN-KO somatic porcine cells5. The team used Sanger sequencing or Capillary Electrophoresis (CE), performed on the Applied Biosystems™ 3130 genetic analyzer, to determine mutations, small insertions or deletions and off-target mutations5. Cells carrying the correct KO were then used for somatic-cell nuclear transfer (SCNT) to generate MSTN-KO piglets5. Interestingly, these MSTN-KO piglets were higher in body weight and displayed increased muscle mass, compared to wildtype5. Such studies can compliment breeding efforts to help increase pork production.
 Genome editing in chickens can be difficult, due to accessibility of the zygote, compared to other organisms6. Oishi et al perform genome editing in chicken using the CRISPR-Cas9 system6. Using the CRISPR-Cas9 system, the team targeted two egg white genes ovalbumin (OVA) and ovomucoid (OVM) in cultured chicken primordial germ cells (PGCs) 6. They used Sanger sequencing to analyze off-target mutations and mutated OVM and OVA genes following genome editing in the targeted PGCs6. The mutant-PGCs were then transplanted into chicken embryos and the team was able to successfully generate OVM mutant chickens6.
Genome editing in chickens can be difficult, due to accessibility of the zygote, compared to other organisms6. Oishi et al perform genome editing in chicken using the CRISPR-Cas9 system6. Using the CRISPR-Cas9 system, the team targeted two egg white genes ovalbumin (OVA) and ovomucoid (OVM) in cultured chicken primordial germ cells (PGCs) 6. They used Sanger sequencing to analyze off-target mutations and mutated OVM and OVA genes following genome editing in the targeted PGCs6. The mutant-PGCs were then transplanted into chicken embryos and the team was able to successfully generate OVM mutant chickens6.
 Creating knockout or knockin models of animals can be very difficult even with access to a simple and powerful tool like the CRISPR-Cas9 systems. Kaneko et al have attempted to make this process simpler7. Using a method called, technique for animal knockout system by electroporation (TAKE), they were able to introduce the CRISPR-Cas9 components into intact mice and rat embryos7. With the help of Sanger sequencing they were able confirm the targeted gene in the offspring7. Such research could potentially be used to help produce genetically modified animal models that may help us study human diseases in the future.
Creating knockout or knockin models of animals can be very difficult even with access to a simple and powerful tool like the CRISPR-Cas9 systems. Kaneko et al have attempted to make this process simpler7. Using a method called, technique for animal knockout system by electroporation (TAKE), they were able to introduce the CRISPR-Cas9 components into intact mice and rat embryos7. With the help of Sanger sequencing they were able confirm the targeted gene in the offspring7. Such research could potentially be used to help produce genetically modified animal models that may help us study human diseases in the future.
Fine tuning technology and screening for a faster workflow
As genome-editing research increases in popularity, it is important to identify fast and cost-effective screening techniques and efficient editing protocols. Liang et al, describe methods for rapid synthesis of the sgRNA and for delivery of the Cas9 protein/gRNA ribonucleoprotein complexes into various mammalian cells either through electroporation or liposome-mediated transfection8. Along with several GeneART™ products, the team used the Applied biosystems™ 3500 genetic analyzer to detect error rates in the synthetic gRNA templates, off-target mutations and on-target indels8. They highlight an efficient two-locus and three-locus multigene targeting technique8. Together, the team was able to present a streamlined workflow that enables gRNA design to analysis of edited cells in ~4 days even in cells that are difficult to transfect8.
When it comes to genome editing, not all transfected cells are edited and hence have to be screened. Screening can be a highly tedious and time-consuming process. Agostino et al identify a rapid, low-cost screening method using the Viia™7 real-time PCR system9. The team optimizes melting parameters to be able to distinguish between wildtype and mutant melting curves and present an approach for massive preliminary screening9. After screening and identifying desired gene-edited cells/embryos, they use the Applied Biosystems™ 3730 genetic analyzer to test the indels and mutations in these cells/embryos to confirm accurate editing9.
Such prior screening can help reduce the number of animal embryos used for research and reduce the number of unnecessary embryos used for downstream sequencing. Such improvements to current techniques has the power to help researchers produce desired gene-edited organisms or cells, in a fast, efficient, and cost-effective manner. Genome editing is a powerful tool that can change how fast we do science. Our complete end-to-end workflow provides guidance on tools, techniques, applications, and technology that can help make this process simple, fast, cost-effective and most importantly, efficient.
For more information:
Learn about Genome Editing with Sanger Sequencing here.
Download the Genome Editing workflow here
Learn more about our Sequencing instruments here
Learn more about CRISPR reagents and products here
For Research Use Only. Not for use in diagnostic procedures.
References:
1] Marraffini LA: CRISPR-Cas immunity in prokaryotes. Nature 2015, 526(7571):55-61
2] Savic N, Schwank G: Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res 2016, 168:15-21.
3] Dong S, Lin J, Held NL, Clem RJ, Passarelli AL, Franz AW: Heritable CRISPR/Cas9-mediated genome editing in the yellow fever mosquito, Aedes aegypti. PLoS One 2015, 10(3):e0122353
4] Ivanov YV, Shariat N, Register KB, Linz B, Rivera I, Hu K, Dudley EG, Harvill ET: A newly discovered Bordetella species carries a transcriptionally active CRISPR-Cas with a small Cas9 endonuclease. BMC Genomics 2015, 16(1):863.
5] Rao S, Fujimura T, Matsunari H, Sakuma T, Nakano K, Watanabe M, Asano Y, Kitagawa E, Yamamoto T, Nagashima H: Efficient modification of the myostatin gene in porcine somatic cells and generation of knockout piglets. Mol Reprod Dev 2016, 83(1):61-70.
6] Oishi I, Yoshii K, Miyahara D, Kagami H, Tagami T: Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci Rep 2016, 6:23980
7] Kaneko T, Mashimo T: Simple Genome Editing of Rodent Intact Embryos by Electroporation. PLoS One 2015, 10(11):e0142755.
8] Liang X, Potter J, Kumar S, Zou Y, Quintanilla R, Sridharan M, Carte J, Chen W, Roark N, Ranganathan S et al: Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J Biotechnol 2015, 208:44-53.
9] D’Agostino Y, Locascio A, Ristoratore F, Sordino P, Spagnuolo A, Borra M, D’Aniello S: A Rapid and Cheap Methodology for CRISPR/Cas9 Zebrafish Mutant Screening. Mol Biotechnol 2016, 58(1):73-78
Thanks for sharing this helpful piece of information. PNA Bio offers the quality Cas9 protein for research purposes.