Cell therapy is an emerging field of medicine that has already made a difference for untold numbers of people suffering from serious conditions. Even with its great potential, scientists in the cell therapy field are facing challenges like complex technologies and high entry barriers. Thermo Fisher Scientific is dedicated to solving these challenges by acting as a knowledge base for researchers, manufacturers, and groups and continually creating and improving technologies to make cell therapy manufacturing easier and more accessible to a wider range of laboratories. Thermo Fisher Scientific also cooperates with industrial and university clients to build state-of-the-art cell therapy manufacturing facilities. Thermo Fisher Scientific’s award-winning Gibco™ CTS™ Rotea™ Counterflow Centrifugation system is one of the ways Thermo Fisher Scientific is making that reality as cost-effective, efficient, simple, and user-friendly as possible.
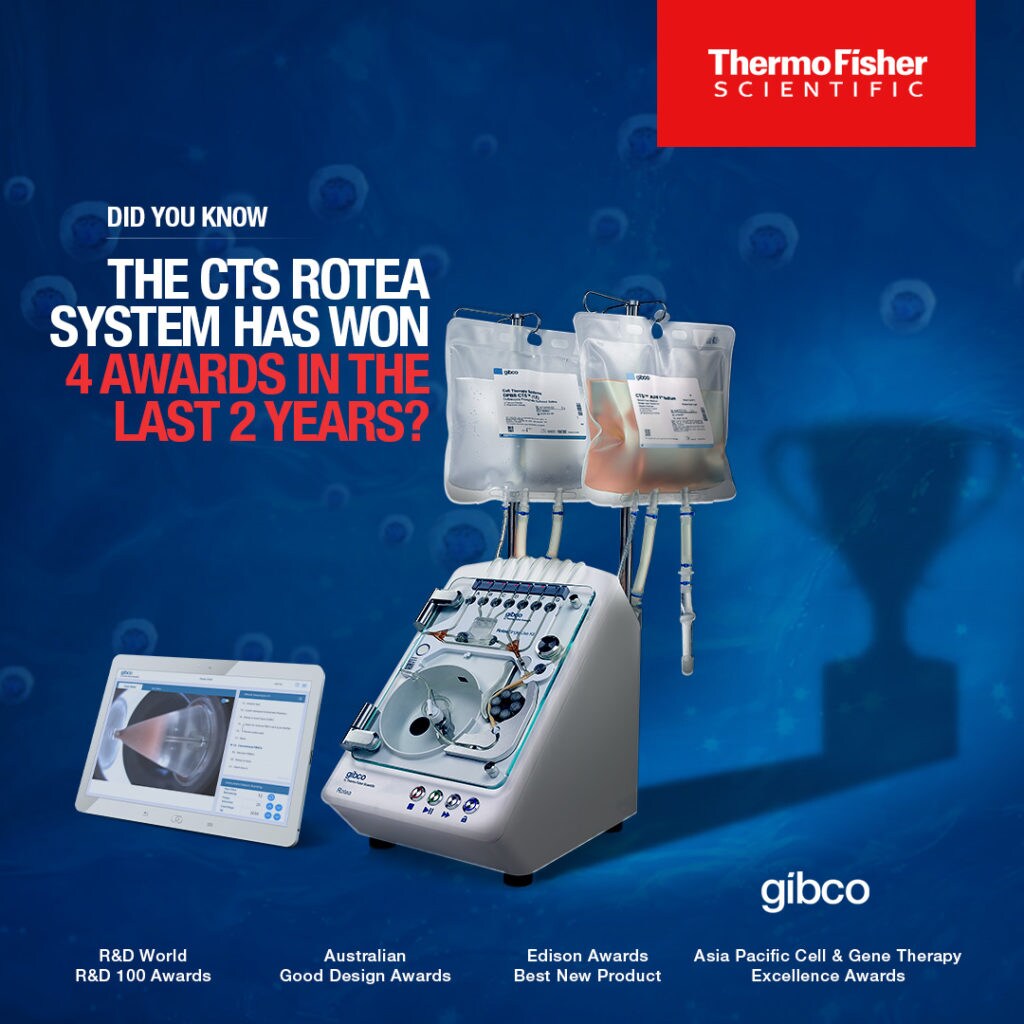
Since its release more than two years ago, the CTS™ Rotea™ System has been recognized four different times for excellence in design and the elegance with which it resolves the challenges that come with cell therapy.
- Thermo Fisher Scientific was recognized as one of Asia’s best of the best in the cell and gene therapy Industry at the 2022 Asia Pacific Cell & Gene Therapy Excellence Awards. The company was awarded the 2022 Best Cell and Gene Therapy Supplier Award – Cell Processing Systems for the CTS™ Rotea™ system. The Asia Pacific Cell & Gene Therapy Excellence Awards pride themselves on recognizing exception pioneers, innovators, researchers and manufacturers in the cell and gene therapy.
- The CTS™ Rotea™ System was named a Silver award winner in the Technology and Tools New Product category at the 2022 Edison Awards. Identified to be among the “best of the best” in its category, Rotea received this honor from the world’s top senior business executives and innovation leaders. Since 1987, the Edison Awards™ have honored excellence not only in new product development but also in service development, marketing, human-centered design, and innovation.
- The CTS™ Rotea™ system earned a 2021 R&D 100 Award in the Analytical/Test category. 2021 was the 59th year for the R&D World Magazine’s worldwide competition. It included entries from 17 different countries and regions and judging by a panel of 40 industry leaders from around the world.
- In 2021, the CTS™ Rotea™ system was selected as a Good Design Award Winner by Good Design Australia. The Australian Good Design Awards have a long legacy of promoting excellence in design and innovation. Since 1958, they have worked to showcase the very best in design and innovation from around the world and across a multitude of industries, including medical and scientific.
Part of the CTS™ Rotea™ system’s design excellence is its special facility for separating useful cells in a mixed volume from other cells while keeping them alive and viable. Conventional centrifuges can separate cells from their fluid medium easily enough but usually result in a dense pellet that can damage or kill cells. This can be useful for biochemistry and genetics studies but not for cell therapy. The CTS™ Rotea™ system uses a constant flow force opposite the centrifugal force to force the centrifuge to be gentle without compromising its effectiveness. By keeping the cells in a fluidized suspension, the CTS™ Rotea™ system can also wash them efficiently and remove dead cells easily. This enables the CTS™ Rotea™ system to deliver more than 90% recovery and viability in a more concentrated final product, exceeding the results of other cell-separation methods used in cell therapy.
Unique to the CTS™ Rotea™ system is the Gibco™ CellCam™ video technology which allows for the visualization of cells live in the fluidized bed through color camera, enabling CFC parameter optimization. The real-time video of the fluidized bed allows customers to easily fine-tune the protocols for a range of applications using the flexible, user-programmable software.
Even more importantly, the CTS™ Rotea™ system is a closed system for cell separation. Cell therapy products require numerous safeguards to be safe for manufacturing use due to the high risk of contamination and the extreme danger that contamination can pose both to the product and to the patient. For open processing systems, this usually means Class A or Class B clean-room spaces in a manufacturing laboratory, which is expensive to set up and maintain and imposes hard limitations on what else can be done with laboratory space. As a closed system, the award-winning CTS™ Rotea™ system can operate in a Class C space, dramatically helping reduce its laboratory footprint without compromising safety. An array of closed system compatible media and reagents assure that use of the CTS™ Rotea™ system helps decrease the risk of contamination. Indeed, the The CTS™ Rotea™ system is part of a broader ecosystem of modular, closed cell therapy instruments from Thermo Fisher Scientific, including the Gibco™ CTS™ DynaCellect Magnetic Separation System, the Gibco™ CTS™ Xenon Electroporation System, and Cell Therapy Systems (CTS™) products , that together are built to help reduce manufacturing risk, support your regulatory submission, help support even the thorniest cell therapy challenges our customers might face, and make CTS the exceptional choice as you transition from the bench to the clinic.
Learn more about the award-winning CTS™ Rotea™ Counterflow Centrifugation System and how it can change the way your lab handles its cell therapy products at thermofisher.com/rotea.
Related links
Download the Cell Therapy Brochure
See all Behind the Bench Cell Therapy blog posts
For Research Use or Manufacturing of Cell, Gene, or Tissue- Based Products. CAUTION: Not intended for direct administration into humans or animals.
© 2023 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.