Want to learn a few basics about macrophages? Want to learn about novel antibody markers that are available to study macrophages? Look no further. Read this blog post.
 Dariusz Stepniak, Ph.D.
Dariusz Stepniak, Ph.D.
Dariusz, a Staff Scientist in R&D at Thermo Fisher Scientific, is a project leader responsible for development and validation of new antibodies. He spoke to us about macrophages and the eBioscience antibodies that could be used to study these cells.
What are macrophages and why are they of interest?
Macrophages, by definition, are a type of immune cells capable of engulfing and digesting various particles in a process called phagocytosis. These cells were first described in 1882 by Russian scientist Elie Metchnikoff, for which he was awarded with the Nobel Prize in 1908. Macrophages are a key component of the immune system. As such they play an important role in protecting the organism against microbial infection. At the same time macrophages are critical for processes of immune regulation, tissue repair and homeostasis. More recently tumor infiltrating macrophages drew much attention due to their role in inducing tolerance and supporting tumor evasion.
Tell me more about macrophages function? What do macrophages do?
Macrophages constitute a very versatile population of phagocytic cells present in most tissues. Besides their direct antimicrobial functions macrophages secrete a wide array of cytokines and chemokines thereby regulating both innate and adaptive immune responses. In addition, macrophages are important in removing dead cell debris, thereby preventing the onset of autoimmune responses. They also play several tissue specific roles, for instance splenic red pulp macrophages (RMP) are critical for clearing old erythrocytes and recycling iron. This unique function is reflected by their high expression of CD163 – a receptor for hemoglobin.
Another good example of their tissue specific function would be the removal of apoptotic cells by retinal macrophages, which is key to protecting the retinal pigment epithelium and retinal ganglion cells. Mutation in the MerTK receptor, carried by these macrophages, results in retinal degeneration and blindness. We have recently developed novel antibodies to both CD163 and MerTK to allow new insights into these processes. Tolerogenic properties of macrophages, however, are a two-edged sword. They help prevent excessive tissue damage and immunopathology but can also create a safe haven for cancerous cells, protecting them from the sweeping arm of the immune system.
Is there a macrophage classification system?
Yes. One popular classification divides activated macrophages into classically-activated macrophages (M1) and alternatively activated macrophages (M2). M2 macrophages comprise a heterogeneous cell population and are often subdivided into M2a, M2b and M2c subsets. In this classification the unpolarized macrophages are often referred to as M0. Although the M1/M2 paradigm played an important role in helping scientists understand the role of macrophages and the mechanisms of their polarization, there is now a growing consensus that this simplistic view of macrophage activation is insufficient in describing the overwhelming versatility of macrophage phenotypes and functions in vivo. Macrophages can be also divided into those that are derived from blood monocytes and those that are derived from embryonic progenitor cells. Distinguishing between these two types is often very difficult and requires dedicated lineage tracing studies.
M1 vs M2 macrophages. What’s the difference?
M1 macrophages are characterized by strong antimicrobial properties and production of proinflammatory factors. M2 macrophages, in turn, constitute a much more diverse and versatile subset including cells involved in anti-parasitic responses, tolerance induction, and tissue remodeling.
Tell me more about M1 macrophages.
M1 macrophages are proinflammatory and constitute a potent arm of the immune system deployed to fight infections. They can express receptors that recognize the pathogen directly (e.g. TLR receptors, scavenger receptors) or indirectly (e.g. Fc or complement receptors). They can also produce reactive oxygen species (ROS) to help kill pathogens. M1 macrophages secrete proinflammatory cytokines and chemokines, attracting other types of immune cells, thereby integrating and orchestrating the immune response. M1 activation is induced by interferon gamma (IFNγ), tumor necrosis factor alpha (TNFα), granulocyte-macrophage colony stimulating factor (GM-CSF), lipopolysaccharide (LPS), and other toll-like receptor (TLR) ligands. The most important transcription factors involved in this response are interferon regulatory factor 5 (IRF5) and signal transducer and activator of transcription 1 (STAT1). The hallmarks of M1 activation are inducible nitric oxide synthase (iNOS or NOS2) expression as well as and high IL-12 and low IL-10 production. Other cytokines secreted by M1 cells include IFNγ, TNFα, IL-1, IL-6, IL-15, IL-18, and IL-23. M1 macrophages express high levels of major histocompatibility complex (MHC), costimulatory molecules, and Fc gamma receptors (FcγR).
Tell me more about M2 macrophages.
M2 macrophages do not constitute a uniform population and often are further subdivided into M2a, M2b, and M2c categories. Common to all three subpopulations is high IL-10 production accompanied by low production of IL-12. One of their signatures is the production of arginase-1 enzyme, which depletes L-arginine, suppressing T cell responses and depriving iNOS of its substrate. M2a macrophages are involved in the T helper cell type 2 (Th2) immune response (i.e., against parasites) and are known to be profibrotic. They are induced by IL-4, IL-10, and IL-13, and are characterized by high surface expression of IL-4R and Fc epsilon receptor (FcεR), dectin-1, cluster of differentiation scavenger receptors CD163, CD206, CD209, and other scavenger receptors. M2b macrophages are considered immunity-regulating and are induced by IL-1, LPS, and immune complexes. Besides IL-10, they also produce IL-1, IL-6, and TNFα. M2c macrophages are induced in the presence of IL-10 and TGFβ. They are often referred to as deactivated or anti-inflammatory, and are known to be involved in driving immune tolerance, tissue repair and remodeling. They produce large amounts of IL-10 and transforming growth factor beta (TGFβ), and express multiple receptors such as CD163, CD206, receptor for advanced glycation end products (RAGE), and other scavenger receptors.
Multiple macrophage phenotypes cannot be readily classified into any of the groups (i.e., macrophages induced during various pathological conditions, such as tumor-associated macrophages). In mice, M1 macrophages can be characterized by expression of iNOS and M2 macrophages by arginase-1 production; however, the expression and function of these markers in humans remains controversial. Therefore, human macrophages from both categories can be characterized by the profile of cytokines produced and the expression of cell surface markers.
What key markers should be considered when characterizing macrophages?
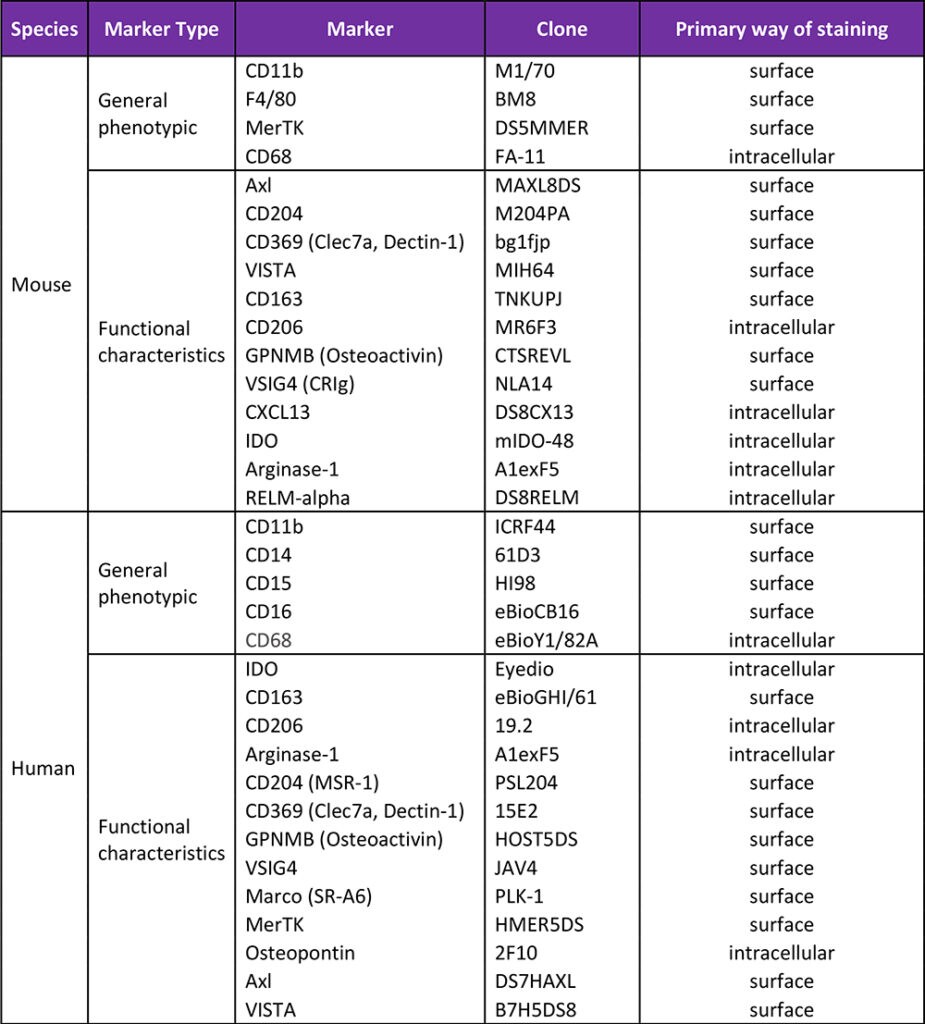
The table below lists markers used to identify macrophages (general phenotypic) and characterize them (functional characteristics). We divided them into surface and intracellular ones although this division is by no means absolute. Instead it reflects the most common way of staining for these antigens. For instance, the majority of CD68 and CD206 molecules are present inside the cell and they are usually being detected by intracellular staining, however, a fraction of their pool can also often be detected on the cell surface. Many other listed markers, such as VSIG4, GPNMB, CD163 or CD204 can be detected both on the cell surface, and intracellularly.
Table 1. Non-exhaustive list of markers to consider when studying macrophages.
Where can I find information about the key antibodies to macrophage markers that are available?
The first resource we recommend is the Thermo Fisher Scientific website. All listed antibodies are presented with a detailed information on their unique staining properties, description of the antigen they recognize, and the staining data on primary human or mouse cells. In addition, three novel antibodies: anti-mouse RELM alpha (clone DS8RELM), anti-mouse CD163 (clone TNKUPJ) and anti-human/mouse Arginase-1 (clone A1eFX5) have been recently discussed in associated blog posts by Dr. Nicolas Schrantz, a Senior Manager of R&D at Thermo Fisher Scientific. In the mentioned blog posts, he shares his insights into the performance of these novel antibodies and their application in the immunophenotyping macrophages using flow cytometry.
Key Macrophage References
Tissue-resident macrophages. Nature Immunology. 2013; 14: 986-995.
Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators of Inflammation. Volume 2015; Article ID 816469, 16 pgs.
Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol. Rev. 2014; 262 (1): 36-55.
The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014; 6: 13 pgs.
Interested in learning about other products or reading about other topics for flow cytometry? Read more Behind the Bench blog posts for Flow Cytometry here.
For Research Use Only. Not for Use in Diagnostic Procedures.
Leave a Reply