The Genetic Insights blog series provides perspectives on the use and impact of genetic analysis in drug development. Genetic research is fueling new discoveries that link diseases, therapies, and patient responses. To learn more, please browse our genetic analysis solutions.
Adeno-Associated Virus for Gene Therapy Research
Why is AAV a Preferred Gene Transfer Vector?
An exciting outcome of the human genome project is the possibility of treating certain genetic disorders by introducing “corrected” genes into affected cells. However, identifying a vector that can efficiently introduce a corrected gene into cells without causing new pathology is a challenge. Adeno-associated virus (AAV) is a preferred gene transfer vector in gene therapy research because it is a nonpathogenic virus with low immunogenicity. AAV vectors are of interest for gene therapy because they can efficiently infect specific target cells that will express the embedded “cargo” genes.
AAV-Mediated Transgene Approach
Advances in Translational Research and Clinical Practice
AAV-mediated gene therapy, or transfer, could potentially be a way to treat diseases caused by defects in individual genes, such as hemophilia, thalassemia, and sickle cell anemia [1]. By April 2021, 245 AAV-based gene therapy trials had been registered in the NIH clinicaltrials.gov database [2]. The FDA approved the gene therapy drugs Luxturna® and Zolgensma® in 2017 and 2019, respectively. Luxturna was developed to treat retinal dystrophy, while Zolgensma was developed as a therapeutic for spinal muscular atrophy. AAV serves as the delivery vector for therapeutic transgenes in both landmark treatments. Their approval is testament to the advances made by adopting the AAV-mediated transgene approach for gene therapy in translational research and clinical practice.
Design and Production of AAV Gene Transfer Vectors
Basic or Translational Research
Highly specialized and rapidly developing branches of research and the service support industry have emerged to support the development and production of AAV vectors. Many academic research institutions have dedicated core laboratories that design and produce AAV gene transfer vectors for basic or translational research.
Brammer Bio was a pioneer in the field with 15 years of experience providing full service and turnkey solutions for the development and production of custom cGMP-grade AAV gene therapy vectors. The company is now a part of Patheon™ Viral Vector Services of Thermo Fisher Scientific [3]. Behind the Bench spoke with Steven Milian, a principal investigator on the Patheon science and technology team, to get an insider view of the role of Thermo Fisher in supporting the field of AAV-based gene therapy. According to Milian, “Providing the customer with expert advice during the product development process in this highly specialized industry and being the first to offer GMP manufacturing are a big part of the company’s success.”
AAV-Based Gene Transfer Vectors – Importance of Simplicity
Why has AAV, once considered a puny bystander, become such a hot and valuable commodity?
One of the striking features of AAV-based gene transfer vectors is the nearly complete reduction of the AAV genome to an essential minimum of a pair of inverted terminal repeats (ITRs). Two ITRs, 145 bases long, in the AAV type 2 expression vector (AAV2) flank approximately 4.5 kb of available space for an exogenous gene cassette, or cargo gene, designed by a researcher (Figure 1).
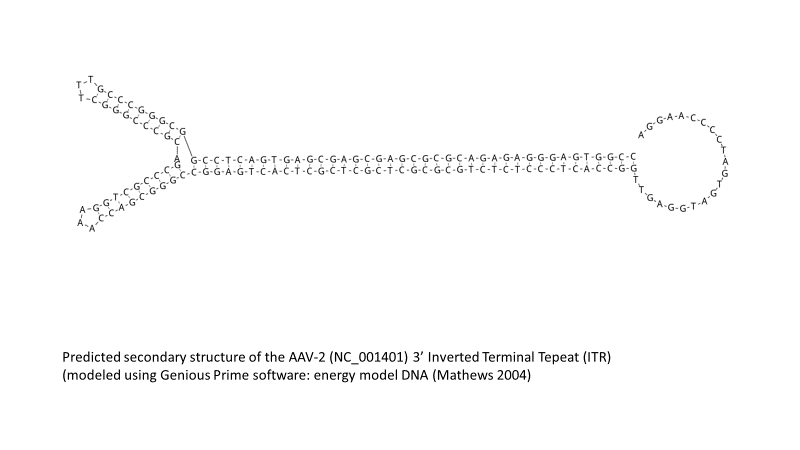
Figure 1. Secondary structure of the AAV-2 (NC_001401) 3′ ITR predicted with a Biomatters™ Geneious Prime™ energy model.
The simplicity of this genetically engineered construct is a big advantage, because no viral proteins are eventually expressed in the target cells. All protein components needed for replication and packaging the “ITR-cargo gene-ITR sandwich” into an AAV capsid vector are provided by transient passage through a host cell that is expressing the necessary replication and capsid factors, either constitutively or by co-transfection of expression plasmids. The final recombinant AAV virus product is then harvested from this cell “factory” and purified to remove host cells and media contaminants. However, AAV-mediated gene transfer cassettes are limited to approximately 4.2–4.5 kb in length. Another part of the AAV vector development and production workflow is ensuring that the ITR-cargo gene-ITR cassette has the desired sequence.
Recombinant AAV (rAAV) Manufacturing and Quality Control
As Steve Milian points out, “The importance of quality control (QC) in the manufacture of recombinant AAV (rAAV) vector preparations cannot be overstated. Genetic analysis methods like qPCR, digital droplet PCR, and DNA sequencing are key to qualitative and quantitative characterization of the product.” Quality control measures, such as sequencing intermediates and final vectors, are therefore critical for success. In the following sections, we briefly describe this approach and the necessary QC steps for successful AAV vector production.
Phase 1 – Setting the Stage: Cloning an AAV Vector Plasmid in Bacteria
Design & Engineer the Cargo Gene
The first steps in constructing an AAV gene transfer vector are rationally designing and genetically engineering the cargo gene (Figure 2).
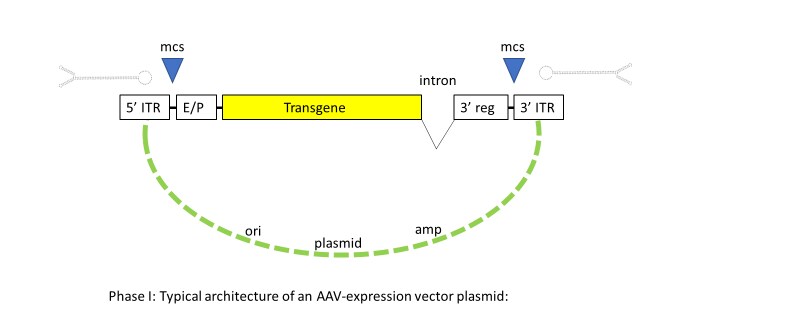
Figure 2. Phase I: Typical architecture of an AAV-expression vector plasmid. The plasmid contains 5’ and 3’ AAV ITR elements and multiple cloning sites (MCS) that can be opened by restriction enzymes for insertion of transcriptional enhancer and promoter sequences (E/P), the transgene to be expressed, intron and 3’ regulatory sequences (poly A), or optional translation enhancer. This recombinant construct is clonally amplified in bacterial culture, purified, and QC-tested before transfection into an AAV-producing host cell system.
This is done by inserting the gene of interest along with the desired regulatory elements between the 5′ and 3′ ITR regions of an AAV plasmid vector, which can include an enhancer for optional cell type specificity, inducible expression, or transcript stability, using standard molecular biological techniques.
Sequencing ITR Regions for Efficient Replication and Packaging
The resulting clones are then grown in liquid culture, and potential recombinants are screened for by restriction mapping and sequencing. Attention must be paid to the ITR regions since the inverted repeat structure can be prone to disruptive mutations or replication errors. The regulatory binding element (RBE) and loop sequences in the ITR regions must be strictly maintained. These are crucial for efficient replication and packaging of the chimeric ITR-cargo gene-ITR construct in the viral capsids during virus production. They also play a critical role in the long-term persistence of the transgene as an episomal element in the nuclei of target cells and the conversion of single-stranded DNA to transcribable double-stranded DNA. Sequencing the ITR regions before producing viral particles is thus a necessary QC step.
Phase II – Metamorphosis: Transformation Into a Finished Recombinant AAV Virus
Recombinant AAV Production, Harvest, Purification, and Quality Control
Once the sequence of the ITR-cargo gene-ITR construct has been verified and deemed suitable for virus production, pure AAV vector plasmid is prepared on a large scale (Figure 3). Suitable host cells that can produce the replication and capsid proteins needed to express the plasmid as single-stranded rAAV DNA, which will be packaged in AAV capsids, are then transfected.
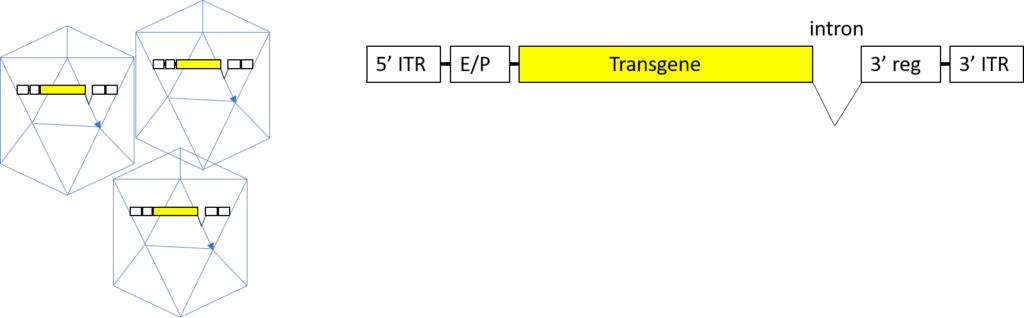
Figure 3. Phase II: Transformation of the transgene construct into a recombinant AAV vector. After transfection of the plasmid AAV construct into a permissive host-cell system for AAV production, the ITR elements and intervening transgene sequences are excised from the bacterial plasmid backbone, replicated into single-stranded DNA, and packaged into AAV capsid proteins. The recombinant AAV is harvested, purified, QC-tested, and ready for in vivo transfer into the final recipient organism.
The recombinant AAV is then harvested and purified. Quality control measures include capillary electrophoresis (CE) or SDS-PAGE analysis to assess purity, measuring capsid and vector genome titers, and verification that the DNA sequence matches the intended design.
Sequencing methods for AAV plasmid DNA quality control
Researchers and service providers have established QC protocols for next-generation sequencing (NGS) during the important phase I pre-transfection and phase II viral output stages. However, given the relatively short segments to be sequenced (<5 kb) and relatively high cost of NGS, Sanger sequencing is an attractive alternative for sequencing the ITRs and insert. Karger et al. presented a poster with an example of a AAV QC protocol [4]. The protocol includes the following:
- A workflow for tackling the most challenging step, which is ITR sequencing with a Sanger method
- An innovative method for restriction mapping AAV plasmid constructs via precision sizing with the Applied Biosystems™ SNaPshot™ Multiplex Kit
- DNA extraction with the Invitrogen™ Dynabeads™ SILANE Viral NA Kit
- AAV viral DNA quantitation with Invitrogen™ Quant-iT™ OliGreen™ ssDNA Reagent
Sanger Sequencing of AAV Plasmid DNA
Sanger sequencing is performed to confirm that the AAV plasmid constructs used to transfect the production cells have the desired sequence before production of the recombinant AAV (rAAV) particles. AAV constructs for gene therapy consist of single-stranded DNA up to 4.5 kb in length that is flanked on both ends by ITRs. In the case of AAV type 2 (AAV2)–based vectors, the left and right ITR are each 145 bases in length. They are rich in guanine and cytosine and are notoriously difficult to sequence [5]. The plasmid construct will contain the transgene and typically includes a promotor and poly(A) termination sequence between the ITRs.
Related: Sanger Sequencing Workflow
Solutions for Your Sequencing Workflow Needs
The Applied Biosystems™ BigDye™ Terminator v3.1 sequencing workflow is ideal for sequencing vector DNA, particularly ITRs. A list of recommended Thermo Fisher products for each step in the workflow is shown in Table 1. The workflow includes PCR amplification of the cargo gene insert and ITRs with M13-tailed PCR primers, hydrolyzing excess primers with the Applied Biosystems™ ExoSAP-IT™ PCR Product Cleanup Reagent, sequencing with the Applied Biosystems™ BigDye™ or dGTP BigDye™ Terminator kit, cleanup with the Applied Biosystems™ BigDye XTerminator™ Purification Kit, and readout on the Applied Biosystems™ SeqStudio™ Genetic Analyzer or Applied Biosystems™ 3500 Genetic Analyzer. Using M13-tailed forward and reverse PCR primers at a final concentration of 200 nM each vastly simplifies the setup of the BigDye Terminator v3.1 cycle sequencing reactions since only standard M13 forward and reverse primers are needed.
Table 1. List of recommended products.
| Product | Cat. No. |
| AmpliTaq Gold 360 PCR Master Mix | 4398881 |
| Platinum II Hot-Start PCR Master Mix (2X) | 14000013 |
| BigDye Terminator v3.1 Cycle Sequencing Kit | 4337456 |
| dGTP BigDye Terminator v3.0 Ready Reaction Cycle Sequencing Kit | 4390229 |
| ExoSap-IT PCR Product Cleanup Reagent | 78200.200.UL |
| BigDye XTerminator Purification Kit | 4376484 |
| SNaPshot Multiplex Kit | 4323161 |
| Dynabeads SILANE Viral NA Kit | 37011D |
Perform Agarose Gel Electrophoresis to Confirm Successful PCR
Given the sequential nature of the PCR and sequencing workflow, it is always advisable to perform agarose gel electrophoresis to confirm that PCR was successful and produced a high-quality PCR product before proceeding to cleanup and cycle sequencing. We used Applied Biosystems™ AmpliTaq Gold™ 360 Master Mix and Invitrogen™ Platinum™ II Hot-Start PCR Master Mix (2X) to amplify the ITR regions in the AAV plasmid construct. We confirmed that a sufficient amount of PCR product was generated despite the high GC content. Both PCR master mixes readily amplified the ITR regions in the plasmid and generated a high-quality template for subsequent BigDye Terminator sequencing.
Standard BigDye Terminator v3.1 sequencing chemistry was designed with an average base composition in mind. It will not enable resolution of the exceedingly GC-rich and repetitive ITR regions, which can be up to 150 bp in length depending on the AAV subtype used. As with other GC-rich sequencing templates, substituting dGTP BigDye Terminator v3.1 reagent for a fraction of the BigDye Terminator v3.1 reagent makes it possible to sequence through GC-rich regions and prevents GC compression in the trace. We suggest substituting 20% to 50% of the BigDye Terminator v3.1 reagent with dGTP BigDye Terminator mix to help resolve the bands in GC-rich regions of the template. Commercially available AAV control plasmids are offered by several suppliers that can facilitate optimization of the sequencing protocol.
Data Analysis
Software Tools for DNA Sequence Analysis and Confirmation
The main goal of sequencing an AAV plasmid construct and rAAV vector DNA is to confirm that there are no deviations from the expected reference sequence. Two software tools from Thermo Fisher are available for this purpose. User-friendly Applied Biosystems™ Variant Reporter™ Software v3.0 and multifunctional Applied Biosystems™ SeqScape™ Software v4.0 are geared towards the expert user. Both advanced DNA sequence analysis tools are included with the purchase of an Applied Biosystems™ Applied Biosystems™ SeqStudio™ Genetic Analyzer. Alternatively, the free variant analysis utility on the Thermo Fisher™ Connect platform (http://apps.thermofisher.com) can be used to align and compare QC sequence data to a reference sequence.
Related: Sanger Sequencing and Fragment Analysis Software
Conclusions
Steve Milian summarizes his motivation and that of his coworkers: “To learn about the successful outcome of a clinical trial of one of our customers using our AAV or lentivirus gene therapy products is so inspiring, just being able to improve one individual’s quality of life dramatically with a single-dose treatment.”
The use of AAV-mediated gene transfer is growing rapidly in basic and translational research as well as clinical gene therapy trials. Having control over the outcome of each step in the somewhat complex journey of rAAV vector production is critical [6,7]. DNA sequencing ensures that the composition of the product output matches the input design.
The Sanger sequencing QC workflow described here works with any Applied Biosystems™ genetic analyzer designed for fragment analysis. The affordable entry-level SeqStudio system should be considered for QC of plasmid vector candidates that will be used for transfection and final rAAV vector production. Alternatively, a 3500 series or high-throughput 3730 instrument can efficiently sequence a large number of clones.
References
- Wang D, Tai PWL, Gao G (2019) Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov 18(5):358–378.
- ClinicalTrials.gov. U.S. National Library of Medicine.
- Patheon by Thermo Fisher Scientific. Viral Vector Services. https://patheon.com/viral-vector-services/
- Poster Karger et al. (2021) http://assets.thermofisher.com/TFS-Assets/GSD/posters/annualmtg_karger-ae_thermofisher_poster.pdf
- Mroske C, Rivera H, Ul-Hasan T et al. (2012) A capillary electrophoresis sequencing method for the identification of mutations in the inverted terminal repeats of adeno-associated virus. Hum Gene Ther Methods 23(2):128–136.
- Thermo Fisher Scientific. Understanding viral vectors. https://www.thermofisher.com/us/en/home/references/gibco-cell-culture-basics/transfection-basics/gene-delivery-technologies/viral-delivery/viral-vectors.html
- Thermo Fisher Scientific. Selecting a viral delivery system. https://www.thermofisher.com/us/en/home/references/gibco-cell-culture-basics/transfection-basics/selecting-a-viral-dna-delivery-system.html
Leave a Reply