While modern methods have streamlined real-time PCR setup and execution, sometimes problems arise and results aren’t what you expected. At Thermo Fisher Scientific, we do as much as possible to take the guesswork out of qPCR and enable improved data quality, making qPCR easier and more informative. To that end, Applied Biosystems™ offers real-time PCR master mixes that include ROX™ reference dye to help improve precision among technical replicates and offer key insights for troubleshooting purposes.
What is ROX dye?
ROX dye is an inert fluorescent dye that can be added as one of the components in a qPCR master mix. Unlike reporter dyes such as SYBR™ Green or FAM™ dye, the fluorescence of ROX dye is not affected by amplification of the PCR product. However, ROX fluorescence is affected by anything else that would alter overall fluorescence readings, such as:
• Bubbles in wells
• Evaporation
• Condensation or droplets
• Instrument issues, such as electrical surges
This allows ROX dye to serve as a passive reference dye that enables fluorescent normalization for qPCR data. To calculate the normalized values (Rn), the reporter dye signal is divided by the ROX reference dye signal. As long as the ratio between ROX dye and the reporter dye remains constant, ROX dye provides a way account for numerous potential variations that would otherwise skew amplification readings.
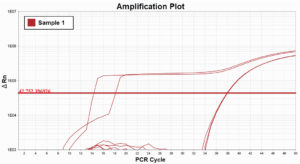
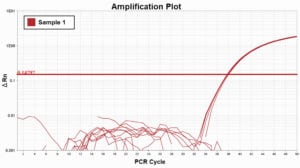
In the example Amplification Plot on the left, you can see how bubbles in wells affect fluorescent readings. However, in the Amplification Plot on the right, we normalized the data using ROX dye as a passive reference and removed any abberations caused by the bubbles.
Bubbles in wells without ROX dye normalization
Bubbles in wells with ROX dye normalization
Why use ROX dye in qPCR?
ROX dye increases precision of technical replicates
Minor variations between individual wells are common when using qPCR assays. And while not required, using a passive reference like ROX dye to normalize data helps achieve a higher level of precision among technical replicates. Without normalization, more replicates may be required to achieve comparable precisions levels, therby increasing the time and resources required for each run. Data normalized with ROX dye is simply more precise than data without it, enabling researchers to run fewer replicates while maintaining statistical power.
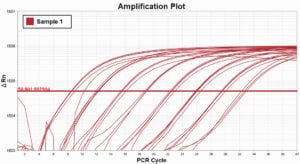
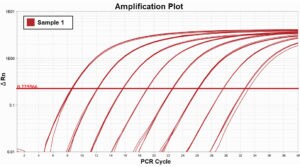
In these example Amplitfication Plots for a cDNA dilution series, you can see how using ROX dye normalization tightens the standard deviation, or precision of technical replicates.
Without ROX dye normalization (STD = 0.028)
With ROX dye normalization (STD = 0.01)
ROX dye help troubleshoot common qPCR issues
ROX dye is also a way to detect the simplest of human errors: forgetting to include the amplification template—that is, the nucleic acid being measured—in the reaction at all. A researcher relayed to us the memorable story of training a friend in qPCR and providing the instructions, “Just put master mix, assay, template, and water in the tube. We’ll see how it amplified tomorrow.” After the reaction failed, troubleshooting revealed the issue. “I could see FAM dye in the multicomponent plot, so that told me that the assay was in there. I could see ROX dye in the multicomponent plot, so that told me that the master mix was in there. I could see plenty of volume in the plate, so it looked like water was in there. I told her that the only thing I couldn’t account for was the template.” At this point, the researcher’s friend admitted, embarrassed, “I didn’t know what you meant by template.”
How do I troubleshoot qPCR using ROX reference dye?
Monitoring ROX fluorescence in the Multicomponent Plot enables researchers to detect various quality control issues with a qPCR ru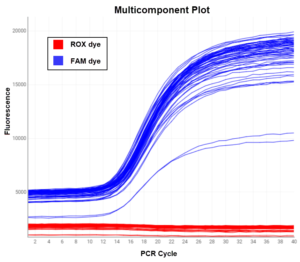 n. To troubleshoot your data using the Multicomponent Plot, review the shape of the ROX dye signal:
n. To troubleshoot your data using the Multicomponent Plot, review the shape of the ROX dye signal:
- ROX dye readings remains flat throughout the run – this is the expected result. ROX fluorescence should stay constant for the reaction duration because it is not affected by amplification, as in this example Multicomponent Plot.
- ROX dye readings increase during a run – this suggests that evaporation is occurring.
- ROX dye readings momentarily spike or drop – this indicates sudden changes that are interferring with how the instrument is recording data in that well, such as air bubbles or an electrical surge.
Just as usefully, ROX dye is not affected by other kinds of errors, such as degraded primers or primer dimer formation, helping distinguish these issues from those related to the equipment or dyes.
Thus, ROX dye is a low-effort tool that provides a way to diagnose problems with a qPCR run and normalize data to make it more precise. Many qPCR master mixes on the market today already incorporate ROX dye, so using it requires no additional effort. Also, real-time PCR software can automatically process ROX dye’s contribution to the run data, saving researchers time to analyze their results. Applied Biosystems™ qPCR master mixes and QuantStudio Real-Time PCR systems are pre-equipped to use ROX dye at all stages of a qPCR experiment to help researchers generate the best possible data. However, a second passive reference dye, Mustang Purple, is also available for researchers who want to have more flexibility in developing a multiplex.
Watch: The Purpose of ROX dye in Real-Time PCR
Read: ROX Passive Reference Dye for Troubleshooting Real-Time PCR Application Note
For Research Use Only. Not for use in diagnostic procedures.
Leave a Reply