The Simple, Sensible & Still Spell Binding Seven Questions About Laboratory Developed Tests
Laboratory Developed Tests (LDTs) and In Vitro Diagnostics (IVDs) are both in vitro diagnostic tests using body fluids (such as blood or urine) or cells / tissues (e.g. pap smear, biopsy) to detect or quantify levels of a desired biomarker. As LDTs and IVDs are both used to diagnose conditions, it is crucial to understand the differences and know the advantages and regulations.
In her webinar, Professor Mara G. Aspinall, Professor of Practice at Biomedical Diagnostics Arizona State University, provides answers to what she refers to as “the simple, sensible, and still spell-binding seven questions about Laboratory Developed Tests”:
- Where do we start: Basics of Diagnostic Test Terminology
- What is an LDT and an IVD?
- Why use LDTs? Why use IVDs?
- Who uses LDTs? Who uses IVDs?
- When can I develop these tests?
- How do LDTs compare to IVDs?
- Can a lab protect their Intellectual Property?
Professor Aspinall’s bright and concise discussion brings a fresh point of view regarding the reasons to develop an LDT or purchase an IVD, and she highlights the advantages of each type of test before deep diving into a detailed overview of what is needed to develop an LDT. Establishing an LDT can seem like a daunting task, but during the webinar Professor Aspinall lays out a clear path for LDT development from lab setup and accreditation, to planning, test configuration, analytical validation, clinical verification, and test launch.
Dr. Aspinall highlights advantages of IVDs
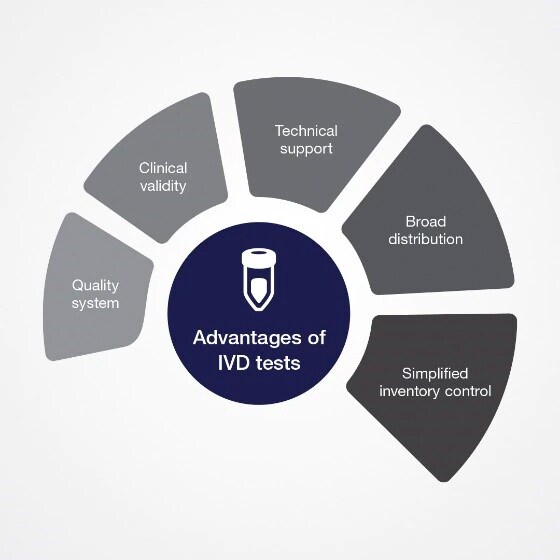
Quality system: Tests are subject to a number of requirements including design controls, manufacturing controls and handling complaints.
Clinical validity: Clinical validation of the test ensures that it detects or measures the specified target analyte, or that it is useful for determining the presence or absence of a clinical condition or predisposition to the condition prior to marketing.
Technical support: Customer can contact supplier technical support to troubleshoot and replace faulty products.
Broad distribution: Many laboratories utilizing the test provide data that can increase (or possibly decrease) confidence in the test. Laboratories performing the same IVD test can report proficiency results to confirm the accuracy of the test.
Simplified inventory control: Users only need to order the manufactured tests for anticipated use, which reduces the amount of documentation required. It requires less time and effort to maintain in-house inventories for IVD tests.
“… Technical support, this is critical, experienced laboratories know there is always going to be problems…”
Throughout the presentation, Dr. Aspinall shares valuable up-to-date information, relevant examples, and insightful reflections to explain and highlight the differences, advantages and use-case scenarios of LTDs and IVDs. Dr. Aspinall ends her webinar by addressing the role of LDTs during the COVID-19 pandemic.
Dr. Aspinall highlights advantages of LDTs
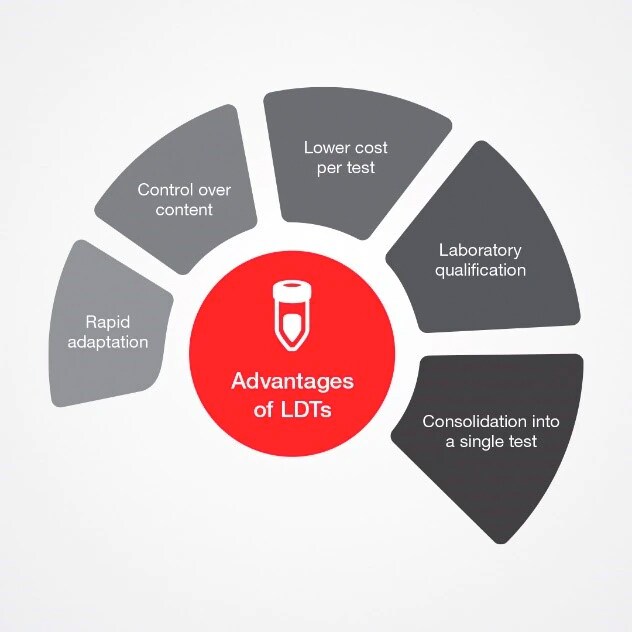
Rapid adaption: LDTs can be developed and modified relatively quickly to respond to market needs.
Control over content: Laboratories can select specific and relevant targets.
Lower cost per test: Technological advances and the availability of off-the-shelf bulk reagents have made complex analyses faster and more affordable.
Laboratory qualification: The laboratory quality management system (QMS) covers all tests, including LDTs, which is not the case for IVD kit production.
Consolidation into a single test: Testing for multiple analytes provides more data per sample and may enable faster diagnosis.
“Number one – and this is really from the laboratory perspective – you own the content. You have control over that content, you can select specific and relevant targets, you can put into that test what you believe is necessary for the clinical market. Secondly, as we talked about, they can be developed quickly, yes, but I think it’s almost more important to say they can be modified relatively quickly.”
Be sure to watch this informative webinar to learn more about the different types of IVD and LDT tests, potential use cases for each, how these tests are developed and regulated, and the significance of LDTs in the midst of a global pandemic.
 Also, don’t miss out on “An introduction to diagnostic testing in laboratories”, published by the College of Health Solutions, Arizona State University. This educational paper covers key aspects of Dr. Aspinall’s webinar, providing in-depth information supported by meaningful examples of LDTs and their different applications.
Also, don’t miss out on “An introduction to diagnostic testing in laboratories”, published by the College of Health Solutions, Arizona State University. This educational paper covers key aspects of Dr. Aspinall’s webinar, providing in-depth information supported by meaningful examples of LDTs and their different applications.
About the speaker
Mara G. Aspinall is a healthcare industry leader and pioneer committed to active civic involvement. Mara also heads the Health Catalysts Group, a consulting firm for HIT and Diagnostics, publishing the popular Health Catalysts Diagnostics Year in Review.
She is a member of the Board of Directors of Abcam, Allscripts, Castle Biosciences, OraSure and Blue Cross Blue Shield Arizona. Mara is an advisor to The Rockefeller Foundation on COVID diagnostics. She is co-author on the Foundation’s national reports on COVID testing education and policy recommendations. She is passionate about education on diagnostics, genomics and personalized medicine. To that end, she co-founded the School of Biomedical Diagnostics at Arizona State University, the first school dedicated to Diagnostics as an independent discipline.
Professor Aspinall was provided an honorarium by Thermo Fisher Scientific for her participation in the webinar. The views expressed are her own and do not necessarily represent the views of Thermo Fisher Scientific.
Explore additional educational content:
Blog: 7 things to know before establishing an LDT
Webinar: Challenges of establishing laboratory developed tests