For a multitude of different diseases, from diabetes to cancer, early intervention is key to limiting morbidity and mortality. In vitro diagnostics that sensitively detect disease-specific biomarkers are central to timely diagnosis and successful treatment.
While IVDs have been powerful for healthcare providers, there are still significant limitations preventing the rapid detection of disease for the global population. These challenges, outlined by Lee and Wong, fall into three major categories, including a lack of:1
- Definitive molecular biomarkers for specific diseases
- Easy and inexpensive sampling methods with minimal discomfort
- Accurate, easy-to-use, and portable platforms to facilitate early disease detection
Salivary diagnostic tests: A solution to IVD’s shortcomings
There is no magical, one-size-fits-all solution for the issues IVDs face. However, the emergence of salivary diagnostic tests – which use whole saliva and other oral sample types for biomarker detection – latter two issues outline above.1-3 Saliva collection and testing can be an easy an inexpensive sampling method with minimal discomfort to the end user, and is both accurate and easy to use as a portable and scalable platform for disease detection. For a multitude of different diseases,
For a multitude of different diseases, from diabetes to cancer, early intervention is key to limiting morbidity and mortality. In vitro diagnostics (IVDs) that sensitively detect disease-specific biomarkers are central to timely diagnosis and successful treatment.
As a diagnostic tool, the use of salivary sample types has several major advantages over traditional and historically favored biofluids, such as blood.
Let’s take a look at the major advantages of saliva collection and the overall benefits of saliva testing.
Saliva provides improved safety through non-invasive collection
The collection of blood, or phlebotomy, is necessary for a huge range of diagnostic tests, making it one of the most common invasive procedures in healthcare.4 Though commonly accepted as a necessary part of diagnostics, when done without the proper procedure, the invasive nature of phlebotomy can lead to the exposure of healthcare professionals to blood-borne pathogens, such as HIV, hepatitis viruses, bacteria, and parasites.2,4
For patients, the use of needles poses several complications. Patients may be uncomfortable with needle use or the sight of blood, which can lead to a variety of vasovagal reactions (such as fainting) in 0.9 to 3.4% of patients.5 More commonly, 14 to 45% of patients experience pain and bruising.5 Lastly, insufficient sterilization before venipuncture can lead to the introduction of microbes directly into the bloodstream.4
Nasopharyngeal swabbing has been the “go-to” sampling method for viral testing since the COVID-19 pandemic began, but one major advantage to saliva collection and testing is that it is a non-invasive collection method. This fact alone is capturing the interest of both the healthcare provider and the end user. As saliva diagnostics has started to be used for SARS-CoV-2 testing, advantages over nasopharyngeal swabbing have become apparent. The latter triggers sneezing and coughing for patients, which increases the risk of viral transmission and causes mild to severe discomfort in patients.6
Saliva is an easier sample type to self-administer in presence of a healthcare provider
Nasopharyngeal swabbing and phlebotomy both require the collection of samples by a trained healthcare professional, foundational training of personnel on how to safely perform collection, a scheduled location and timing for collection, and the use of PPE to limit exposure to pathogens.4
The collection of saliva is much easier, more flexible, and introduces the option of convenient self-collection by a patient while in the presence of a health care provider. Decentralized collection and expanding test sites enable the sampling of a wider and more diverse population. Recently, a variety of saliva collection devices and testing methods have proven effectively facilitating scalable, routine testing for a broad population, an important practice for consistent monitoring of ongoing viral spread.12
Better Stability and Transportability
As mentioned above, using saliva as a sample type allows decentralized self-collection, even in areas without access to a medical system. As long as there is a way to transport samples from a patient’s residence to a testing facility, a decentralized testing system provides significant advantages. With SARS-CoV-2 testing, many emergency authorized tests have implemented at-home kits that use saliva collection methods and several studies have demonstrated the stability of SARS-CoV-2 RNA in saliva, under a variety of storage conditions.13-15
This would be much more challenging to implement using blood. Transportation of blood samples and excessive handling can lead to lysis of red blood cells, which can skew lab results.16 Fluctuations in temperature, prolonged transport times, and overall transport quality can also alter diagnostics results.17
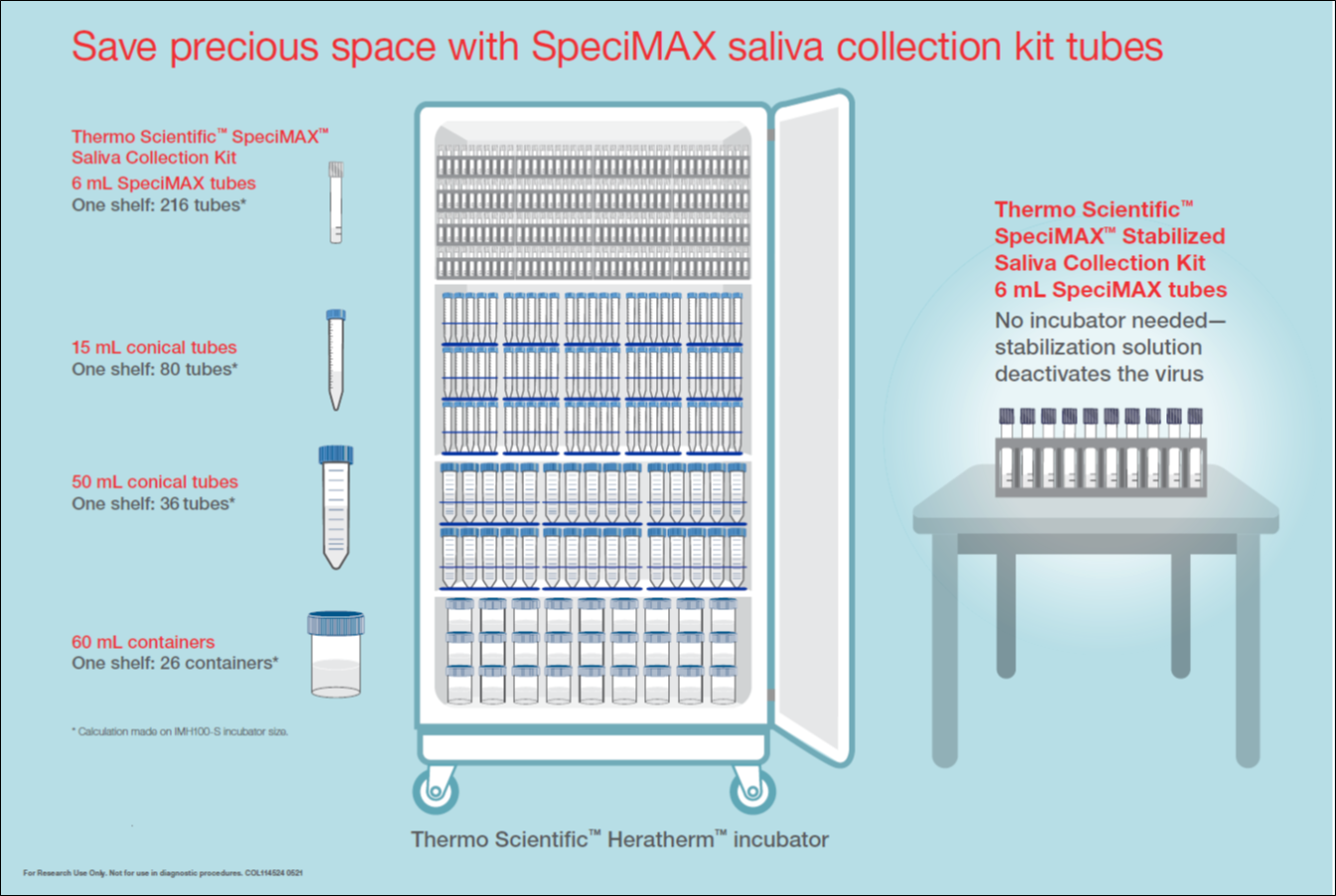
SpeciMAX Dx Collection Kits can be stored effectively in standard 6mL test tubes, 15 and 50 mL conical tubes, and 60 mL containers. Save space while keeping samples safe in the Thermo Scientific Heratherm incubator.
Saliva sampling is an affordable solution compared to blood or nasopharyngeal
Several studies have reported the cost-effective nature of saliva sampling compared to blood sampling.8,18 The price of extraction and purification of select salivary biomarkers is also lower: For commercial extraction of DNA from saliva, the cost is $6.93 per sample versus $10.88 per sample for blood.8
In the case of SARS-CoV-2 testing, saliva testing is equally sensitive as nasopharyngeal testing, yet much less costly, saving up to $636,105 per 100,000 persons sampled.19-21
Accurate detection of oral and systemic disease biomarkers
Saliva is a complex biofluid with a vast array of diverse biomarkers present.22 One disadvantage to its use as a diagnostic sample type is that many potential analytes are present in 100- to 1000-fold lower concentrations than in blood, putting significant pressure on the sensitivity of the IVD technology for accurate detection and diagnosis.23,24
But the number of salivary biomarkers for oral and systemic diseases is increasing and sensitive, selective methodologies have aided in their detection. DNA, RNA, and protein biomarkers associated with cancer, periodontal disease, autoimmune diseases, viral and bacterial diseases, neurodegenerative diseases, and cardiovascular diseases have been identified in saliva. With the growing use of genomics, transcriptomics, proteomics, and other “omics” techniques to study saliva, the discovery of others is on the horizon.1,22
While regulatory agencies haven’t yet approved tests in many of these disease areas, commercially available, saliva-based diagnostics are available for substance abuse, HIV, and HPV-related oral cancers. These widely used tests act as important validation for ongoing development and commercialization efforts.22-24 In addition, several studies have demonstrated that saliva diagnostic tests for SARS-CoV-2 are highly concordant with tests that use nasopharyngeal swabs.19-21
Bringing the benefits of saliva testing to broader populations
Thermo Fisher Scientific is working towards streamlining saliva sample collection and creating kits that make saliva testing as efficient, rapid, reliable, and secure as possible.
Thermo Fisher Scientific has launched SpeciMAX Dx Saliva Collection kits to make sample collection convenient and downstream analysis fully scalable through automated instruments including KingFisher Specitrax and KingFisher Flex. Automated DNA and RNA extraction improve laboratory workflows by reducing touch-time and maintaining repeatable results. Areas that can be automated include test tube de-capping, sample transfer and pipetting, and standardized or customized protocols for washing, lysing and eluting samples.
Contact a Thermo Fisher representative to see how our single-use collection tubes can help you implement a fast, reliable, and scalable saliva testing program.
This article is for Research Use Only. Not for use in diagnostic procedures.
References:
- Lee YH, Wong DT. Saliva: an emerging biofluid for early detection of diseases. Am J Dent. 2009;22(4):241-248.
- Lee JM, Garon E, Wong DT. Salivary diagnostics. Orthod Craniofac Res. 2009;12(3):206-211.
- Salivary Diagnostics. NIDCR website: https://www.nidcr.nih.gov/grants-funding/funded-research/research-investments-advances/salivary-diagnostics. Published August 1st, 2018. Accessed October 8th, 2021.
- WHO Best Practices for Injections and Related Procedures Toolkit. Geneva: World Health Organization; March 3rd, 2010, Best practice in phlebotomy and blood collection. Available from: https://www.ncbi.nlm.nih.gov/books/NBK138496/
- Stevenson M, Lloyd-Jones M, Morgan MY, et al. Non-Invasive Diagnostic Assessment Tools for the Detection of Liver Fibrosis in Patients with Suspected Alcohol-Related Liver Disease: A Systematic Review and Economic Evaluation. Southampton (UK): NIHR Journals Library; February 2012. (Health Technology Assessment, No. 16.4.) Appendix 8, Diagnostic venepuncture: systematic review of adverse events. Available from: https://www.ncbi.nlm.nih.gov/books/NBK97517/
- Saliva sampling using the SpeciMAX Dx Saliva Collection Kit. Thermo Fisher Scientific website: https://assets.thermofisher.com/TFS-Assets/BID/Application-Notes/saliva-sampling-specimax-saliva-collection-kit-app-note.pdf. Published July 26th, 2021. Accessed September 20th, 2021.
- Bahlo M, Stankovich J, Danoy P, et al. Saliva-derived DNA performs well in large-scale, high-density single-nucleotide polymorphism microarray studies. Cancer Epidemiol Biomarkers Prev. 2010;19(3):794-798.
- Abraham JE, Maranian MJ, Spiteri I, et al. Saliva samples are a viable alternative to blood samples as a source of DNA for high throughput genotyping. BMC Med Genomics. 2012;5:19.
- Viltrop T, Krjutskov K, Palta P, Metspalu A. Comparison of DNA extraction methods for multiplex polymerase chain reaction. Anal Biochem. 2010;398(2):260-262.
- Zhang L, Kirchhoff T, Yee CJ, Offit K. A rapid and reliable test for BRCA1 and BRCA2 founder mutation analysis in paraffin tissue using pyrosequencing. J Mol Diagn. 2009;11(3):176-181.
- Ambrosone CB, Ciupak GL, Bandera EV, et al. Conducting Molecular Epidemiological Research in the Age of HIPAA: A Multi-Institutional Case-Control Study of Breast Cancer in African-American and European-American Women. J Oncol. 2009;2009:871250.
- Allicock OM, Petrone ME, Yolda-Carr D, et al. Evaluation of saliva self-collection devices for SARS-CoV-2 diagnostics. Preprint. medRxiv. 2021;2021.02.01.21250946.
- The 7 Best At-Home Coronavirus Tests of 2021. Healthline website: https://www.healthline.com/health/at-home-coronavirus-test. Published April 21st, 2021. Accessed October 9th, 2021.
- Ott IM, Strine MS, Watkins AE, et al. Simply saliva: stability of SARS-CoV-2 detection negates the need for expensive collection devices. Preprint. medRxiv. 2020;2020.08.03.20165233.
- Griesemer SB, Van Slyke G, Ehrbar D, et al. Evaluation of Specimen Types and Saliva Stabilization Solutions for SARS-CoV-2 Testing. J Clin Microbiol. 2021;59(5):e01418-20.
- WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy. Geneva: World Health Organization; 2010. Executive summary. Available from: https://www.ncbi.nlm.nih.gov/books/NBK138659/Zaninotto M, Tasinato A, Padoan A, et al. An integrated system for monitoring the quality of sample transportation. Clin Biochem. 2012;45(9):688-690.
- Daksis JI, Erikson GH. Heteropolymeric triplex-based genomic assay to detect pathogens or single-nucleotide polymorphisms in human genomic samples. PLoS One. 2007;2(3):e305.
- Bastos ML, Perlman-Arrow S, Menzies D, Campbell JR. The Sensitivity and Costs of Testing for SARS-CoV-2 Infection With Saliva Versus Nasopharyngeal Swabs : A Systematic Review and Meta-analysis. Ann Intern Med. 2021;174(4):501-510.
- Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of Saliva and Nasopharyngeal Swab Nucleic Acid Amplification Testing for Detection of SARS-CoV-2: A Systematic Review and Meta-analysis [published correction appears in JAMA Intern Med. 2021 Mar 1;181(3):409]. JAMA Intern Med. 2021;181(3):353-360.
- Vogels CBF, Brackney DE, Wang J, et al. SalivaDirect: Simple and sensitive molecular diagnostic test for SARS-CoV-2 surveillance. Preprint. medRxiv. 2020;2020.08.03.20167791.
- Roi A, Rusu LC, Roi CI, Luca RE, Boia S, Munteanu RI. A New Approach for the Diagnosis of Systemic and Oral Diseases Based on Salivary Biomolecules. Dis Markers. 2019;2019:8761860.
- Saliva: An Often Forgotten, but Convenient Diagnostic Fluid. AACC website: https://www.aacc.org/cln/articles/2013/january/saliva. Published January 1st, 2013. Accessed October 11th, 2021.
- Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ, Wong DT. Salivary biomarkers: toward future clinical and diagnostic utilities. Clin Microbiol Rev. 2013;26(4):781-791.




Leave a Reply