
In the life sciences, consistency and reproducibility are king. For cell biologists, neither is possible without accurate measurement of cell confluency.
Despite being a critical and routine determination in the cell culture workflow, confluency can become a highly subjective measurement when “eyeballed” by individual researchers…or even the same researcher on different days.
Luckily, there are alternatives to visual confluency measurement that researchers can use to help improve process and reproducibility across experiments.
What is cell confluency?
Cell confluency is a routine measurement used to track cell proliferation during cell culture. It is not the absolute cell number, but rather the percentage of culture dish or flask area covered by adherent cells.
Cell confluency is a crucial parameter that helps researchers determine timing for passaging, transfecting, or harvesting for downstream applications like drug treatments, cell therapies, induced pluripotent stem cell (iPSC) work, differentiation experiments, and more. Essentially, it’s a non-negotiable step in maintaining healthy cell cultures and ensuring accurate experimental results.
Overconfluency in particular can lead to cell stress or death as culture nutrients deplete, and cells begin competing for physical space. Cell behavior in a crowded environment may misrepresent natural gene expression, growth, or morphologies. Lysing cells will also release cytotoxin debris, and overconfluent cell cultures in general are more susceptible to fungal and bacterial contamination – issues that can irretrievably ruin your culture.
Why is accurate cell confluency important?
Even at low to medium cell confluency levels (i.e. <80%), an accurate and consistent estimation has many benefits, for example:
- Consistent Results: Matching confluency across experiments ensures that cells are at the same growth stage, which helps reduce variability in results and improve reproducibility – particularly in industry and manufacturing applications.
- Efficient Workflows: Researchers can better plan each workflow step, avoiding resource and time waste. Better accuracy can mean avoiding the loss of cell stocks and mistakes in freezing and thawing at the wrong stage of growth.
- Maximum Efficacy: Certain procedures like transfection or drug treatment are more effective at specific confluency levels. Overconfluency may also muddy interpretation in applications like drug discovery, where it can be difficult to determine the origin or non-specific effects.
What are the different options for measuring cell confluency?
With all this in mind, it should come as no surprise that precise control and measurement of cell confluency are vital for conducting reliable experiments — yet the leading method of estimating confluency percentage is still good old fashioned “guesstimation” involving manually inspecting the culture under a microscope.
As you might imagine, inconsistencies are common with the visual approach. While measures of 50% or 100% confluency can be relatively easy to estimate, most of the time researchers are looking for the 70% or 80% confluency signaling high cell viability and proliferation — and these guesses are much trickier to nail. One researcher’s “70% confluent” may be another’s “80%.” Just a ten percent variability from the true value has potential to significantly skew experimental results.
Some researchers use chemical dyes and plate readers for a quicker, more quantitative indirect measurement, but these dyes can be destructive to cultures.
How accurate is your visual confluency estimation?
Consider the iPSC microscopy images below – before scrolling down the page, note your visual confluency estimates for each frame. If you’d like, also ask a colleague to estimate so that you can compare values and differences between them. (Keep reading for actual confluency values later in the post!)
Estimate the confluency of the iPSC culture images below:
Image #1
Image #2
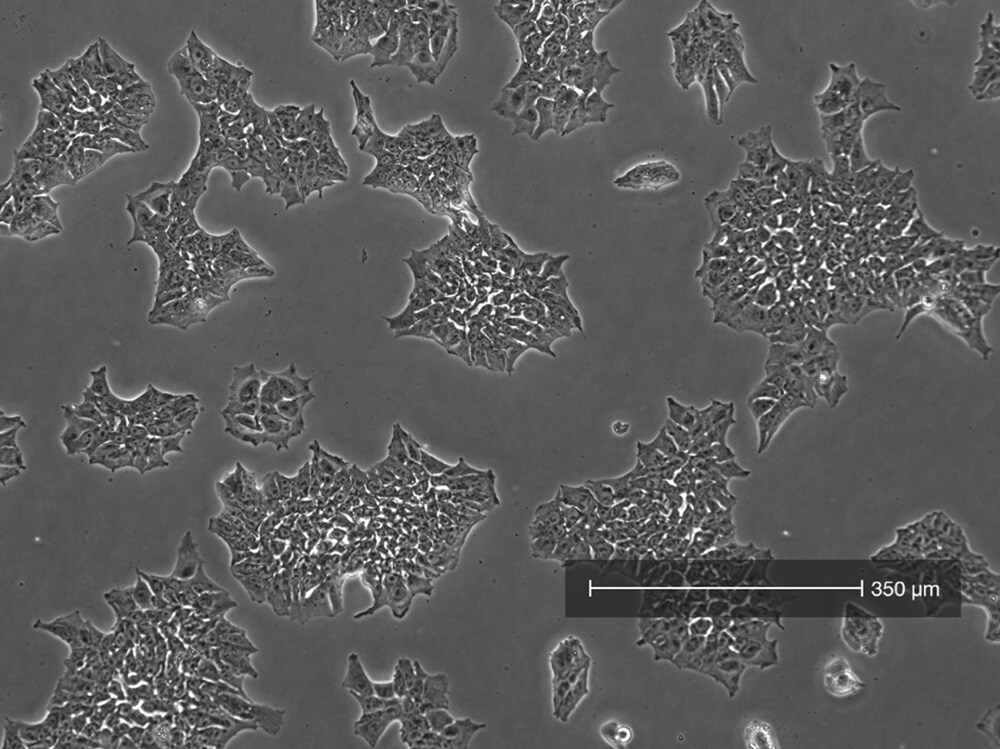
Image #3

Image processing tools can make confluency measurement faster and more consistent
Modern image processing tools offer a less subjective, more consistent framework for assessing confluency. While manual image processing options exist, these tools typically use thresholding, edge detection, deep learning algorithms, and more to automatically differentiate cells from the background of a high-quality microscopy image. From there, the software can calculate the percentage of total area covered by cells.
To date, most of these tools have involved separate equipment pieces or processes for image capture and image analysis for confluency.
The newly launched EVOSTM M3000 Imaging System streamlines the process as the first-to-market with real-time, automated cell image analysis capability. It will be a good choice for researchers who need to do more with less (time, equipment, space); the EVOS M3000 has options to enable both brightfield and fluorescent applications while also merging the imaging and confluency analysis steps in one go. Its small footprint is also ideal for small standard hoods.
The difference between visual and automated confluency measurements
What kind of difference can automated confluency measurement do for you and your lab? Grab your answers from the image questions above and see how they compare to the real-life EVOSTM M3000 Imaging System assessments below:
Answers
Image #1: 32% confluency

Image #2: 52% confluency

Image #3: 85% confluency

How did you do? If you find that you have room to improve, check out more about automated confluency at the jump below.
» Learn more about real-time automated confluency measurement
Want to read more stories like this? Subscribe to Connect to Science, your portal for life science news.
##
© 2024 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.
For Research Use Only. Not for use in diagnostic procedures.

Leave a Reply