Search Thermo Fisher Scientific


Method Lifecycle Management Overview
Comprehensive solutions for method development, method knowledge, and method transfer coving the lifetime of your drug product monitoring
What is Method Lifecycle Management?
Method Lifecycle Management (MLCM) is a control strategy ensuring that as long-term methods evolve, they perform as originally intended throughout their lifetime.
Changes in production materials, analytical instrumentation, consumables, or modifications to the drug product can have an impact of a validated method to continue to meet their original criteria.
Challenges also extend to inter-laboratory method transfer either internally or to outsourcing partners such as contract research organizations (CROs). It is critical that from development and analytical method validation, through to method transfer and long-term performance monitoring that continuous knowledge of the method is captured and used to asses the impact of any changes in the future such as a change in manufacturing process or a change in the analytical column used for separation.
Back-to-school: explore and modernize gradient chromatography separations

Analytical Target Profile (ATP)
ATP underpins all stages and states the requirements of the analytical procedure at all stages, such as accuracy and precision. The ATP is driven by the known CQAs of the product.
Method qualification
Method qualification requires robust technology and software to give confidence the developed method will perform as expected over an extended period.
Method design and development
Method design and development requires an upfront knowledge of the product's critical quality attributes (CQAs), often by the implementation of Analytical Quality by Design (AQbD). Instruments are required to have flexibility in use to facilitate parameter adjustments, and automated features built in to both speed up, and ease the burden of screening method parameters and consumables.
Method verification
Method verification requires long term monitoring of the method, including when changes happen such as method transfer or a change in production. Implementing new technology typically allows for more improved methods, but the impact on legacy methods must be minimal and monitoring of CQAs is not compromised.
Thermo Fisher Scientific Analytical Technologies for the Pharmaceutical Industry - Anthem Video
At Thermo Fisher Scientific, our pharmaceutical analytical technologies ensure you get accurate results, day after day, run after run.
We deliver confidence in production control and lot release testing to ensure that drugs are safe and of a consistently high quality.
Our software and chromatography capabilities, built for regulated environments, provide operational simplicity, verifiable performance, and quality by design.
Method development, transfer, and validation
Method development
Automated column and eluent screening option
Screen up to 13 eluents by equipping your Vanquish Method Development HPLC and UHPLC system with a solvent selection valve for extensive, automated screening.
Automated, software-assisted method development
Automate, simplify, and accelerate method development with leading software packages such as ChromSwordAuto Chromeleon Connect or Fusion QbD software.
Automated HPLC method development and robustness
Develop and test method robustness easily using automated software with analytical quality by design (aQbD) principles.
Fast and robust UHPLC method for metolazone and related impurities
Significantly reduce the number of experiments, method development time, and related costs.
UltiMate 3000 and Vanquish Core HPLC instruments for the compendial analysis of commonly prescribed drugs
This application note is intended to demonstrate the usability of Vanquish Core and UltiMate 3000 HPLC instruments for the straightforward application of compendial methods.
ICH guideline update: Method lifecycle
The International Council for Harmonisation (ICH) has updated its guidelines Q2 and added Q14 to cover method lifecycle from development to validation with the emphasis for analytical scientists to focus on incorporating a quality mindset through method lifecycle.
Technology to simplify method transfer
Thermo Scientific Vanquish Core HPLC System enables easiest method transfer
Confidently transfer methods from other labs or even other equipment seamlessly with the use integrated tools and software. Our method transfer tools allow you to duplicate other HPLC vendors’ system settings to more easily replicate methods, such as those from a Waters Alliance HPLC system, Agilent 1260 Infinity LC system and Shimadzu Nexera-i system to a Vanquish Core HPLC system.
Method validation and secure data storage
Automate design and execution of robustness testing using ChromSword AutoRobust Chromeleon Connect and accelerate your method validation using the workflows available in the Chromeleon 7 Extension Pack ICH templates. The Chromeleon Data Vault ensures secure data storage and accessibility for users within the CDS infrastructure. With Vanquish Method Development HPLC and UHPLC systems, all data generated is safely backed up in Chromeleon CDS Data Vault, enabling easy data exchange throughout the organization.
Enhance your capabilities with the pharmaceutical applications compendium
Case studies
Industry stories and solutions
Maximizing laboratory productivity
How modernizing an instrument park leads to significant reduction in system suitability failures.
Adopting new LC instruments within an existing network
Deployment of the Vanquish UHPLC system at Patheon sites across the globe has enabled improvements in assays.
Vanquish Core HPLC system cost of ownership
By improving system performance, efficiency, and impact on wearable parts and consumables, more time can be spent running samples.
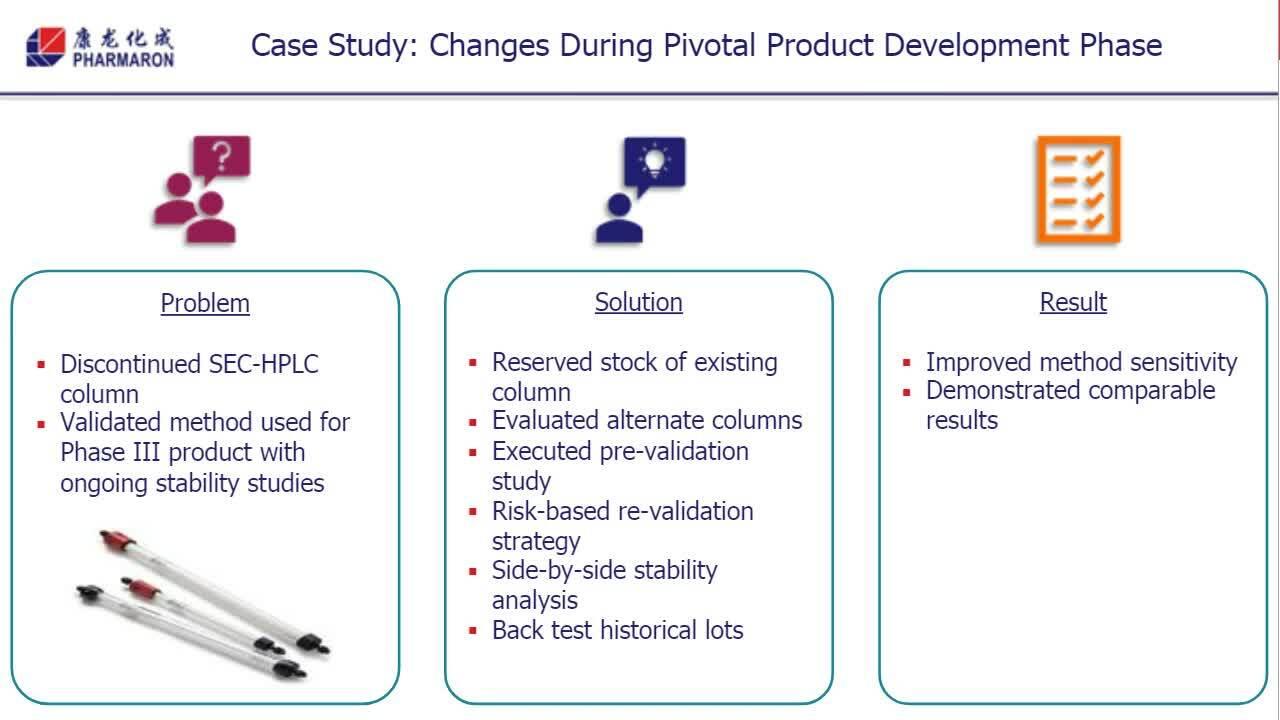
Equipment and Method Lifecycle Management – Expert roundtable
In this roundtable discussion, experts from Pharmaron UK will introduce the analytical method lifecycle and why it is needed.
Overcoming the challenges of HPLC method transfer
Method modernization is often less difficult than the common perception, and good practices can be put in place to streamline and facilitate the process.
Key products
Advanced LC systems for solving tough analytical challenges
Obtain new benchmarks in accuracy, precision, and sensitivity with the Thermo Scientific Vanquish HPLC and UHPLC systems. With state-of-the-art quaternary or binary high-pressure solvent blending, these ultra-high-performance liquid chromatography systems share all Vanquish values, such as a design focused on uptime, robustness, and reliability. Built-in tools for seamless method transfer and long-term performance to ensure you have the confidence to run any assay without compromise.
Vanquish Flex UHPLC Systems
Succeed with flexibility. Vanquish Flex UHPLC systems excel from method development to routine analyses. With binary and quaternary pump options, the Vanquish Flex UHPLC systems offer outstanding performance for LC and LC-MS based nitrosamine workflows.
Vanquish Core HPLC System
Eliminate disruptions to your routine analytical work with the Vanquish Core HPLC system. Switching to Vanquish Core is simple and seamless. A full suite of method transfer tools, including tune-able gradient delay volume, makes adoption easy. Like all members of our Vanquish product line, Vanquish Core HPLC systems offer hardware precision and simplicity of operation. Ideal for routine nitrosamine impurity analysis.
- Separate more peaks than ever before. The Vanquish system improves specifications on all fronts. It supports higher backpressures, better thermostatting, optimized volumes, better linearity, and more sensitivity.
- Regain time during your projects. The system improves analysis speed, increases sample capacity and has improved robustness.
- Operational simplicity of the Vanquish system provided by optimized design and automated features.
Add mass spectrometry (MS) to your IC and LC analyses for access to the valuable data that only MS can provide
The easy-to-use Thermo Scientific ISQ EC Single Quadrupole Mass Spectrometer seamlessly integrates with ion chromatography (IC) and high performance liquid chromatography (HPLC) systems. Both novices and experts alike can quickly master MS to gain more insights from every sample.

ISQ EC Single Quadrupole Mass Spectrometer
Gain more insights
- Higher sensitivity and more accurate quantitation
- Chromatographic peak mass confirmation, eliminating false negatives and false positives
Resolve complexity
- Improved resolution of analytes in complex matrices
- Better selectivity
- Resolve co-eluting peaks using their mass-to-charge ratio
Explore how mass spectrometry provides reliable verification and quantification ›
Explore near-universal response along with the best performance, reliability, and flexibility in HPLC and UHPLC detection
Thermo Scientific Vanquish Diode Array Detectors (DAD) and Multiple Wavelength Detectors (MWD) detectors offer excellent linearity and optimized noise performance, and feature an ultra-wide dynamic range to allow detection of highly concentrated main compounds alongside trace-level impurities.
In addition, the Vanquish DAD HL uses Thermo Scientific LightPipe technology to achieve the best signal-to-noise performance through a combination of low baseline noise, a very long light path, and minimal dispersion.

Thermo Scientific Vanquish Diode Array Detectors (DAD) and Multiple Wavelength Detectors (MWD)
- Industry leading linearity with up to 2.7 AU
- Reduced interfering refractive index and thermal effects means less baseline drift and more reliable peak integration
- Data acquisition at rates of up to 250 Hz using up to 10 absorption channels and one spectral field delivers the best support of ultrafast separations
- Intuitive user interaction, convenient flow cell and lamp usage tracking, and integrated wavelength validation through a holmium oxide filter contribute to ease-of-use
Unlike mass spectrometry, which requires an analyte to form gas phase ions, and UV detection, which depends upon the nature of the analyte’s chromophore, charged aerosol detection is a near universal detector.
The different selectivity between the three methods can be observed in the chromatograms on the left, which were generated using all three detection techniques simultaneously with a single injection. The results of charged aerosol detection are at the top, mass spectrometry results are in the middle, and UV detection results are at the bottom
Explore the benefits of CAD coupled with an HPLC or UHPLC system ›
Quality columns you can rely on for quality results
Thermo Scientific HPLC and UHPLC colums are rooted in over 40 years of leading LC column technology to help you achieve the most accurate and reliable results from your HPLC analyses. Our extensive family of products offers a variety of particle sizes and column designs to meet all separation needs, including improved resolution, enhanced sensitivity, faster analysis, and consistent performance.
Because experiments, analytes, and budgets are different, we have organized our portfolio of SureSTART autosampler vials and closures into three performance levels to provide you with the affordability, compatibility, and performance you need.
Additional resources
Development of a robust pH gradient method
Learn more about monoclonal antibody charge variant analysis using cation exchange chromatography and a quality-by-design approach.
Automated method optimization
Learn more about drug-to-antibody ratio determination using hydrophobic interaction chromatography.
HPLC Method Transfer-A CDMO perspective
Two Analytical Development Lab Chemists will share key learnings, feedback and best practices for transferring methods from competitive instruments and developing new ones onto the Vanquish liquid chromatography platform.
The Successful Leap of Faith for an Established Chromatography Lab
Changing existing LC equipment in a regulated lab to a entirely new provider involves method transfer and solving challenges.
Panel Discussion: Making Instrument Equivalency Assessments and Actions Successful
Panel discussion on instrument equivalency and method transfer in CDMO environment when changing to a new instrument vendor.
Phase Appropriate Analytical Equipment and Method Lifecycle Management: Expert roundtable discussion
Experts from Pharmaron UK introduce analytical method life cycle approaches and provide two case studies on the topic.
Analysis of impurities in topiramate by HPLC
Case Study - Quantifying more with less: Implementing charged aerosol detection to improve drug safety
Contact us
* Required field