Search Thermo Fisher Scientific
Intact Protein Analysis Information

Intact protein characterization using mass spectrometry
Comprehensive characterization of biotherapeutics is necessary to satisfy safety standards set by regulatory agencies and helps to ensure protein drug efficacy. Intact protein analysis using a combination of liquid chromatography (LC) and mass spectrometry (MS) provides information on the accurate mass of the protein and the relative abundance of its isoforms. The analysis of these complex heterogeneous protein biotherapeutics benefits from high resolution accurate mass (HRAM) instrumentation. Combining HRAM with powerful data deconvolution algorithms facilitates structural confirmation and accurate identification of protein modifications.
Intact mass analysis and native mass spectrometry
Biopharmaceutical characterization is required throughout all stages of drug development and manufacturing. During drug development, liquid chromatography–mass spectrometry (LC-MS) approaches are used to structurally characterize monoclonal antibodies (mAbs) produced using cell lines. The main objective is to determine monoclonal cell populations producing the desired mAb with a high degree of fidelity and activity for a specific antigen. The analysis of intact biologics is continued throughout manufacturing to assess purity and heterogeneity characteristics for lot release purposes.
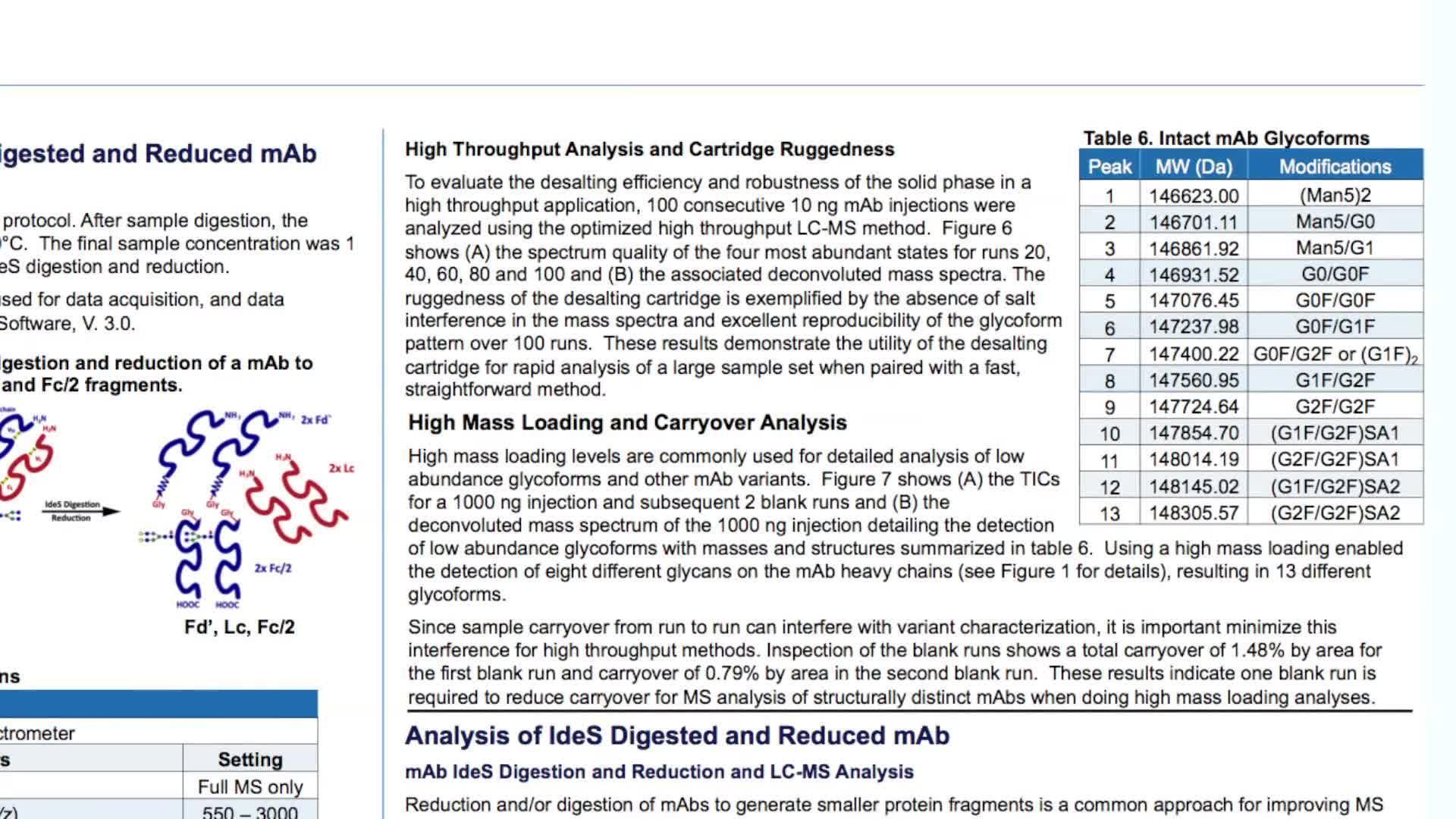
Multiple analytical approaches can be utilized to assess biotherapeutic molecules in their intact state. Information on accurate protein mass and heterogeneity can be provided by MS analysis following a short desalting step and is important for quantification of the various glycoforms and protein variant analysis, important considerations for therapeutic drug formulation and storage. Biotherapeutic drugs can be analysed at their subunit level following reduction and/or digestion. Analysis of therapeutic antibody subunits facilitates exact molecular weight analysis of their heavy and light chains and further top down analysis can provide sequence confirmation.
Protein analysis in native or native-like conditions decreases charge state value resulting in mAb detection at higher m/z ranges with more spatial resolution. For example, native mass spectrometry conditions also allow the preservation of structurally-critical non-covalent bonds which may be necessary to the analysis of cysteine-linked antibody-drug conjugates (ADC)
Featured intact protein analysis learning content
Poster video
Join mass spectrometry expert Dr. Stephane Houel as he presents this poster on the characterization of antibody drug conjugates using intact protein mass spectrometry, at the American Association of Pharmaceutical Scientists (AAPS) convention.
Poster note
In this poster note we report the characterization of ADCs with enzymatic labeled antibody N-glycans, using the Thermo Scientific Q Exactive Plus and Orbitrap Fusion mass spectrometers.
Poster video
Join biopharmaceutical chromatography expert Dr. Shanhua Lin as she discusses a poster detailing novel approaches for antibody subunit characterization, at the HPLC 2016 convention.
Poster note
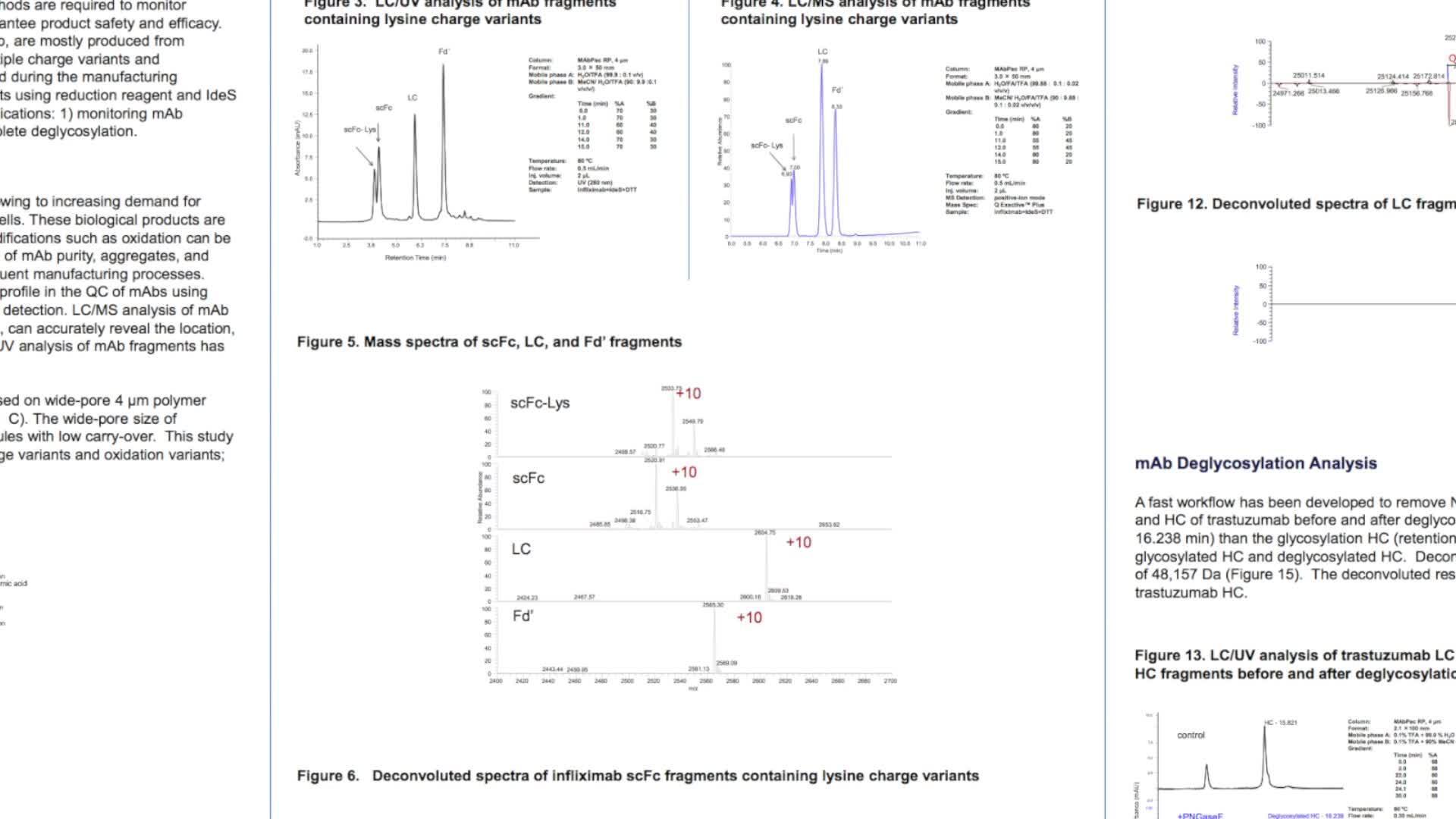
In this scientific poster monoclonal antibodies (mAbs) are broken down into several large subunit fragments using reduction reagent and IdeS enzyme. A fast LC/MS separation method is employed for two applications: confirming complete deglycosylation, and monitoring charge plus oxidation variants.
Poster video
Join chromatography expert Dr. Shane Bechler as he presents a simple method for rapid purification of therapeutic proteins by mass spectrometry from high salt solutions in this fast and reproducible workflow.
Poster note
Mass spectrometry is an essential tool in the characterization of mAbs. However, the sample matrix typically comprises a variety of salts, stabilizers, detergents, and other adduct forming ions. Find out how to rapidly remove these interfering species in this poster.
Intact protein analysis literature library
No records were found matching your criteria