Search Thermo Fisher Scientific
Considerations When Purchasing a Flow Cytometer

Now more than ever and across vast areas of study, researchers are developing innovative applications for new areas of research, improving data quality, and expanding the boundaries of experimental limitations. These advances are enabled by increasingly sophisticated instrument design and technology—especially in flow cytometry. Researcher demand is driving the evolution of instrumentation throughout the market, resulting in advanced flow cytometers designed to fulfill the needs and budgets of all types of labs.
Learn about key experimental concepts and flow cytometer components including:
- Comparing different hardware parts found in most flow cytometers
- The importance behind technologies for measuring light from fluorescence-labeled cells
- Distinguishing flow cytometer fluidic and optical system design technologies that best suit key applications such as immunophenotyping, difficult sample types including tumors and sticky cells, high-volume needs for rare events, and specific research goals
Objective information on comparing technical specifications, evaluating system fluidics and optics, and engaging in key technical conversations enables principal investigators, core facility managers, and researchers considering many different brands or just one, key insights for informed buying decisions.
Investigating flow cytometry instrument capabilities to maximize your research
The 40-page Flow Cytometer Evaluation Guide will enable a better understanding of the components and capabilities of various flow cytometers for objective comparison of instruments from several manufacturers.

Fluidics hardware in a flow cytometer
Flow cytometry interrogates single cells or other particles suspended in fluid for multiparametric analysis of properties. The flow of cells or particles gives a distinct advantage over other methods such as imaging and PCR by collecting information from single cells in a sample. Sophisticated technology is required for a sample to enter the instrument and be delivered to the optical system.
The importance of having a good fluidics system

The power of flow cytometry is the ability to quantitate the physical characteristics of individual cells in a heterogeneous population. This process requires the fluidics system to deliver large numbers of cells into the flow cytometer in order to produce a statistically significant amount of data. The flow of cells into and through the instrument must be consistent for accurate counting and measurements.
Coincidence in high-speed flow cytometry

Figure 1. Coincidence rate. (A) One cell is excited by the laser beam. (B) Two cells are excited by the laser beam producing a doublet signal. Cells in doublets and triplets can mask the true biological nature of a sample.
Cells enter into the flow cytometer in an unaligned, arbitrary fashion. Cells ideally flow past the interrogation laser in single file for individual detection, but there are some instances where the cells flow through together (as governed by Poisson distribution or samples with cell clumps). When there is more than one cell in the laser path at the same time, this is called coincidence.
Two cells simultaneously passing through the laser can lead to difficulty in distinguishing the cells of interest. If one cell is positive and the other is negative, and they both pass together through the laser, the instrument will read the event as a positive signal. When this occurs, the data obtained from this event is a doublet event and not usable. The technology behind the focusing system provides the mechanism for single-cell interrogation in the optical system.
Fluidics delivery systems

Figure 2. Systems for sample entry into the flow cytometer. (A) Pressure-based fluidics rely on the pressure difference between the sample fluid stream and the sheath fluid surrounding the sample stream. (B) Volumetric-based fluidics provide the ability to obtain a concentration and count measurement since the exact sample volume can be measured.
Pressure-based and volumetric-based fluidics are two common mechanisms for cells to enter into the flow cytometer.
Samples with large volumes of cells can quickly enter the instrument with pressure-based fluidics. A pressurized system is created by a specialized tube forming a seal with the instrument. The sealed tube initiates a difference between the pressure of the sample and of the instrument sheath fluid to move cells into the optical system. Pressure-based systems allow for smooth sample delivery, larger sample volume, and less risk of running out of sample. Backpressure is a problem with this type of system, and it can clog if it is not properly maintained.
Volumetric-based fluidics enable more flexible cell delivery. The mechanism for this system measures a precise volume of sample and injects it into the instrument. Benefits include the ability to determine absolute cell count and accommodate difficult samples, including sticky cells or large cells, by being less sensitive to clogging from backpressure. This system has added value as any type of tube or plate can hold sample and deliver it into the system.
Sample delivery pumps

Figure 3. Two types of pumps. (A) Peristaltic pumps move fluids using a rotating-head system. The rollers in the head depress the tubing and drive the fluid forward. (B) The syringe pump draws up a sample and injects it into the fluidics system.
Sample delivery in pressure- and volumetric-based systems is accomplished by using either peristaltic or syringe pumps.
Peristaltic pumps are a common, low-cost alternative for less-expensive instruments. Fluidics systems equipped with these pumps employ a series of rotating rollers to press on the tubing. This type of system can quickly move large sample volumes. This type of technology, however, does not provide accurate cell count as the amount of sample delivered into the instrument fluctuates with the rotation of the pump.
Volumetric delivery systems rely on a syringe pump in order to precisely control and measure sample volume. Syringe pumps provide the mechanism for absolute cell counting and sample recovery. Returned sample can be used for other experiments such as PCR and imaging or stored to run again. Volumetric sample delivery represents a quality, well-crafted designed for precise delivery of cells into an instrument.
Technology to flow suspended cells into the optics system

Figure 4. Cell focusing systems.
Single cells flowing through the optical system can be obtained using different methods of fluidics in an instrument.
Most flow cytometers use traditional fluidic focusing technology such as hydrodynamic focusing. This technology uses the difference in pressure and speed between the sheath fluid and cell sample to move a cell forward.
Other fluidics systems use acoustic-assisted hydrodynamic focusing. This focusing method is a modification of the traditional system and uses sound waves to focus cells into a middle of the flow cell and traditional hydrodynamics to deliver the cells to the optical system. Acoustic-assisted hydrodynamic focusing reduces fluid usage while maintaining the benefits of hydrodynamic focusing. The combined use of acoustic focusing and sheath fluid aligns cells into a single file, resulting in some experiments running at quicker speeds with lower coincidence rates. The small amount of sheath fluid has an added benefit of keeping the flow cell clean and less prone to clogging. This type of technology can flow dilute samples quickly.
Sample handling
Pressure-based systems use tubes and mostly process one sample at a time. For high-throughput applications, these flow cytometers can be equipped with a separate multiwell sampler. Multiwell plate sampling may not be available in some models as specialized tubes are required to create the pressure differential for samples to enter into the flow cytometer.
Some volumetric-based flow cytometers have the capability to sample from both tubes and multiwell plates. The pipette-like syringe both mixes and injects the sample into the instrument.
Plate samplers can be attached to robotic handlers for high-throughput processing of many multiwell plates. These systems handle large volumes of samples by having greater fluidic support to continuously run multiple plates.

Optics options for a flow cytometer
The capabilities of an optics system in a flow cytometer will directly influence how an experiment is designed and executed. Lasers provide the light to excite fluorophores conjugated to antibodies or reagents for use in the detection and analysis of various components and aspects of the cell. Detectors are the devices that capture the light of specific wavelengths, amplify, and resolve this light to provide the information of the specific physical characteristic details the fluorophore is meant to measure. The technology behind the lasers and detectors dictates the number and type of fluorophores used in an experiment. This is why it’s important to understand the options of the many components that create the optical system in order to obtain optimal data.
The importance of having a good optics system

The identification of a cell and its physical characteristics is dependent on the power of the laser beam and the sensitivity of the detection system. Most experiments require multiple fluorophores to tag cell surface and intracellular proteins in order to identify cell populations, cell cycle, population doublings, apoptosis, and cell viability. Good optics systems with quality components will capture the full-range of biologic and biochemical properties of a cell.
An optical system with sensitivity and range is important as fluorophores emit light in a color spectrum
Laser excitation and emission wavelengths of fluorescent dyes

Figure 5. Laser excitation and emission wavelengths of fluorescent dyes. (A) Cell with multiple fluorescent labels. (B) PE and FITC are both excited by the blue 488 nm laser and have overlapping emission spectrums. (C) PE is excited by the yellow 561 nm laser, but FITC is not. (D) Alexa Fluor 647 dye is excited by the red 640 nm laser.
Each fluorophore has an excitation energy range and a specific light-emitting spectrum. Cells can be labeled with multiple fluorescence-labeled antibodies, fluorescent dyes, and fluorescent proteins. Lasers emit power at specific wavelengths to provide the different amounts of required excitation energy.
Spectral overlap (fluorescence overlap)

Figure 6. Double excitation maximums. (A) PE can be excited with the 488 nm laser. (B) PE has another excitation maximum at 561 nm. Excitation of PE by this laser more efficiently emits fluorescence.
Some fluorophores have multiple excitation wavelengths of different efficiencies. For example, either a blue 488 or yellow 561 nm laser can excite the fluorophore PE. A blue 488 laser excites PE at a lower energy level. A yellow 561 nm laser provides more excitation to the fluorophore and results in a greater amount of emitted photons. Stronger emitted light may resolve a small, dim population of cells that might not have been seen on an instrument without a yellow 561 nm laser.
Optics component technology
Lasers
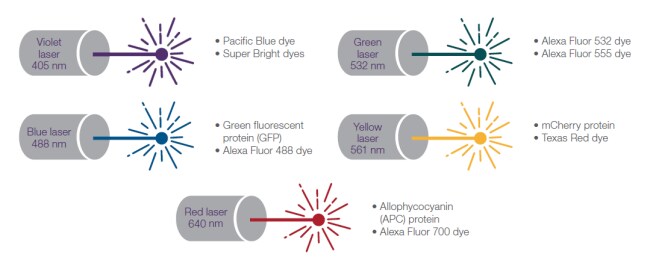
Figure 7. Laser wavelengths with common fluorescent proteins and select dyes.
Commercially available flow cytometers may include violet (405 nm), blue (488 nm), green (532 nm), yellow (561 nm), and red (640 nm) lasers. Base instruments typically include the blue 488 nm laser. Adding more lasers provides the option to increase the number of fluorophores that may be used in one experiment. Violet, blue, yellow-green, and red lasers are sufficient to excite most fluorophores used in multi-fluorophore experiments. Some flow cytometers can be equipped with a UV laser in the 350 nm range to spread out the excitation and emission wavelengths when designing multicolor experiments.
Laser profile

Figure 8. Laser alignment. (A) Cells receive full laser power with properly aligned lasers. This ensures each cell will have similar energy for fluorophore excitation, providing less data variation. (B) Laser misalignment may cause uneven distribution of energy for fluorophore excitation. This may increase the chances for data variation.
Every cell should receive similar laser excitation energy in order to compare emitted fluorescent signals between cells in an experiment. Consistent laser beam output with correct laser alignment helps provide an optimal signal to every cell that passes through the interrogation point. Well-aligned laser beams result in data produced by biological, not instrument, variation.
In samples with weakly labeled cells, suboptimal excitation energy can lead to negative results. When laser power is not consistent, there will be more variation between collected emissions from cells resulting in greater statistical error. Shifts in laser alignment may provide less or unequal excitation energy to each cell passing through the beam.

Figure 9. Laser profiles. (A) Gaussian emission profile. Photons are dense and most intense in the center. (B) Flat-top beam intensity profile covers a larger and more flat area. Cells pass through a wider apex to generate fluorescence.
Gaussian laser beams are found in most flow cytometers. Cells must pass through the center of the beam for optimal excitation energy.
Recently developed flat-top laser profiles cover more area to excite fluorophores. The wider apex allows cells in a sample to have an even application of energy to help produce consistent results.
Laser arrangement

Figure 10. Laser arrangement. (A) Collinear laser arrangements have multiple lasers arranged to focus on a single spot of the interrogation point. (B) Spatially separated lasers are individually arranged and focused along the interrogation point.
Multiple lasers in one instrument are most commonly arranged in two formations. The arrangement affects both the number of fluorophores used in an experiment and also the detection of cell fluorescence.
Collinear spatial arrangement fires all lasers at the same time to focus laser light onto one area, resulting in the simultaneous excitation of many different fluorescent dyes.
This laser arrangement is less flexible in multicolor panel building as multiple fluorescence signals are simultaneously emitted and require greater compensation. A collinear laser arrangement works well with lasers of two different wavelengths that excite fluorescent dyes with minimally or non-overlapping emission spectra. Less expensive flow cytometer instruments may have this type of laser arrangement to save on component cost.
Spatially separated arrangement sequentially fires lasers on the cell stream. Cells stained with multiple fluorophores pass through each laser beam one at a time for separate excitation and detection of emission spectra. This type of arrangement provides flexibility for the number of dyes used in one experiment. The individually emitted fluorescence signals provides higher sensitivity and requires less compensation. Large, multicolor panels can be more readily built using this arrangement as it generates significantly less fluorescence overlap in similar emission spectra.
Fluorescence separation components

Figure 11. Fluorescence emission. (A) Excited fluorophores emit light in a limited range of wavelengths. (B) Some fluorophores can have overlapping emission spectra.
Excited fluorophores emit light in a limited spectrum, not at a specific wavelength. Emitted fluorescence needs to be passed through optical filters before it is captured and analyzed. Without fluorescence separation, the unfiltered light from multiple fluorophores would overlap and be difficult to analyze

Figure 12. Components for fluorescence separation. (A) Optical filters divide the emitted signal from fluorophores based on the fluorescence wavelength range. (B) Prism monochromator arrays separate light into regions of different wavelength and focus light from these regions onto different detectors.
Cells labeled with multiple fluorophore-conjugated antibodies can be analyzed because of the development of optical filters. These light separation components are the most commonly used for fluorescence separation in flow cytometers as they are easily accessible and user interchangeable. Combining different filters allows for varying color combinations to create both small and large multicolor panels. This is a tried-and-true system with durable components. Look for a system with easily changeable filters.
Spectral flow cytometry is different from conventional flow cytometry. Instruments with this technology use prisms to focus emitted fluorescence in the visible light spectrum. Light emitted from multiple fluorophores will overlap and requires a second step with complex algorithms for separation. The benefit of this technology is that it expands the total number of color channels from fewer lasers. This technology is still developing for flow cytometry, and computational fluorescence separation maybe limited for certain fluorophores.
Emission detectors

Figure 13. Components for emission detection. (A) PMTs capture and multiply signal emitted by a fluorophore. (B) APDs capture photons on a semiconductor and then multiply the signal.
The emitted fluorescence from a single cell is a weak signal. The light needs to be converted into an adjustable form of data for analysis. An emission detection device captures fluorescence light and converts the light waves into an electronic signal. This step is important as individual electronic signals can be amplified and the data can then be processed by the user.
Photomultiplier tubes (PMTs) are detection devices found in the majority of flow cytometers. Multiple PMTs are arranged at the end of the optical path and capture each filtered fluorescence signal. This type of detection device is useful with multicolor panel experiments because PMTs can capture a dynamic range of fluorescence, from very dim to very bright signals in a linear manner from the broad range of the spectrum from UV to IR.
Alternative detection devices include the avalanche photodiode (APD) to conduct and multiply electrons generated from emitted fluorescence. Experiments that heavily use fluorophores emitting in the far-red spectra can benefit from APDs. These detectors efficiently capture signals emitted in the red and near- infrared spectral regions.
Voltage settings

Figure 14. Voltage settings. (A) Positive signal emitted from a fluorophore-labeled cell is not in the detectable range and is off scale because the PMT voltage is too high. (B) The voltage is lowered and the positive signal is now detectable.
Sample background may be detected as autofluorescence from cells or other components of a sample running on a flow cytometer. Fully visualizing the physical characteristics of stained cells requires capturing the full range of negative (dim) signal through positive signal. In other words, the ideal PMT voltage must be adjusted to view the maximum separation between the associated stained fluorophore and the cell’s detectable (negative) autofluorescence.
Before running cell samples, PMT voltages of the flow cytometer should be optimized using a fluorescent standard to calculate the optimal voltages for each PMT.
A PMT voltage may be adjusted if necessary, especially if the positive signal is too bright. Users may adjust the voltage by using unstained control cells as a negative for background signal. Lowering the voltage of the PMT allows proper measurement of the positive signal and decreases the intensity value of the negative population.
The amount of signal from fluorescence light detected by the flow cytometer is controlled by voltage settings. Setting a higher or lower voltage produces more or less gain on the PMT detector for proper fluorescence signal measurement.
Locked voltage settings are part of simple flow cytometer function, thus simple flow cytometers offer a limited range of detectable emitted fluorescence. Adjusting voltage settings can be challenging since the user needs to be able to distinguish positive cell populations from background. New users may over- or under-adjust voltages. To help these users, voltages are locked for a small panel of fluorescent dyes and their associated detectors. Instruments with locked settings simplify the process by limiting the ability to change voltage settings.
Unlocked or adjustable voltage settings are preferable for users running multicolor panel experiments. Locked voltage settings are preset to detect fluorescence emitted from a limited panel of fluorescent dyes. Adjustable voltage settings are not limited to a set panel of fluorescent dyes. This feature allows users to employ larger panels or include more types of dyes. Adjustable voltage settings also allow users to check their positive, single-stained controls to place their PMT signals on scale. PMT voltages may also be adjusted for optimal signal compensation as part of multiparameter flow cytometry experiments.
Fluorescence compensation

Figure 15. Compensation. (A) Overlapping emitted signals can produce false-positive data points. (B) Compensating data results in the removal of the overlapping emitted fluorescence. The compensated data points will produce more accurate results.
Emission from multiple fluorophores produces a range of light in the visible spectrum. Data collection from multicolor panels will produce overlapping fluorescence signals. This can be problematic as positive single-labeled cells may appear as double-positive cells.

Figure 16. Compensation techniques. (A) Traditional compensation uses well-researched sets of algorithms analyzing data from fluorophores, negative gating, or unstained controls to process overlapping emission into a single color. (B) Spectral unmixing uses sets of algorithms such as constrained Poisson regression or nonnegative matrix factorization to process light into a single color.
Compensation is the separation of signal from each fluorophore by a set of algorithms. Traditional compensation separates specific wavelengths. The process measures each fluorophore individually and then uses algorithms to create a matrix of correction values. These corrections are applied to the spectral overlap to produce individual values. This process straightforwardly and quickly computes signal compensation.
Spectral unmixing is different from traditional compensation in that it provides the spectrum of emitting light versus a single wavelength.
Channels and the number of fluorophores in an experiment
Figure 17. Channels. (A) A one-laser system can have multiple channels. (B) The emitted light overlaps and requires compensation. (C) A 3-laser system will have more channels because there are more detectors. (D) The channels are spread out, allowing for more fluorophore options and less compensation.
Components to capture emitted light in both PMTs and APDs are called channels. The number of fluorophores detected by a flow cytometer will be determined by the number of available channels.
A one-laser system can have multiple channels. This means a single laser can excite multiple fluorophores, and the detectors can capture emitted light at different wavelengths from a sample. This type of experiment requires complex compensation as all fluorophores can emit light in overlapping wavelengths.
Multiple lasers may be of benefit since they provide more channels. Additional channels confer easier panel design as there are more fluorophore choices and less compensation. This may allow for an experiment with better resolution of populations.

Instrument maintenance
Flow cytometers are sophisticated instruments designed to last many years. Most components are permanent fixtures. Periodic maintenance and routine cleaning will minimize parts replacement and help provide high-quality data.
The importance of instrument cleaning and maintenance

Figure 18. Common contaminants from improper maintenance. (A) Carryover is the contamination of cells or particles from previous samples. (B) Dyes binding to nucleic acid are sticky and can create background noise. (C) Microbes can grow in fluidic bottles and other parts of a flow cytometer.
One type of problem that arises when regular instrument cleaning is not performed cleaning is the accumulation of material from previous samples.
Carryover cells from previous samples are a problem for sensitive experiments such as measurement of rare cells. The rate of false positives increases when carryover cells are present. Flushing between samples or after experiments can reduce the number of cells or particles leftover from a previous experiment.
Regular cleaning prevents buildup of fluorescent dyes; certain dyes such as nucleic acid dyes are more prone to build up. Collections of sticky dyes, cells, and debris create more opportunity for tubes to be clogged. Instruments that run cell culture or tissue samples have a higher chance of bacterial contamination. Signs of contamination include cloudy fluid in the waste bottle and a high number of event collections in blank samples. Running cleaning solution or bleach through the system according to the manufacturer’s instructions can discourage microbe growth.
Routine cleaning

Figure 19. Daily cleaning. (A) Run bleach and DI water between samples. Some instruments can automatically clean. (B) Startup and daily shutdown cleaning procedures help prevent buildup of dyes and carryover of cells. (C) Additional daily maintenance checks may involve automated protocols with software guidance.
Minimize contamination between samples with cleaning. Between each user experiment, most manufacturers advise users to run 10% bleach then DI water before running the next experiment. Some instruments have automatic cleaning between samples.
Most systems require distilled water and cleaning solution before use and at the end of the day to remove cells and dyes from flow cytometer tubes and flow cells. Startup and shutdown cleaning protocols are detailed in most user manuals. Daily maintenance helps ensure fluidic tubes and flow cells are free from contamination with microbes or old sample.
Some flow cytometers have additional daily maintenance requirements including fluid tank preparation, a check for air bubbles trapped in filters, and emptying waste containers. Other flow cytometers have automated protocols with software guidance. Automated systems reduce user responsibility by removing certain tasks such as air bubble checks or shutdown procedures. These protocols are in place to save time and provide accurate cleaning.
Fluidics decontamination

Figure 20. Fluidics maintenance. (A–F) Clean or replace flow cytometry instrument parts to maintain normal instrument performance and minimize carryover, background noise, and microbial contamination.
A combination of fluidics flushing, changing of sheath filters, and deep instrument cleaning can remove carryover, fluorescent dyes, and microbes. Most flow cytometers require a deep cleaning of sheath tubing, waste tubing, fluidic containers, and the flow cell every 2 weeks to one month. This prevents the buildup of debris and removes biohazardous material and microbes.
Depending on the type of fluidic system, components may have to be removed and replaced or cleaned . Peristaltic systems require that tubing be removed and replaced every 6 months to a year. Without replacement, contaminants or debris will appear during event collection. Pressure systems require regular maintenance of nozzle tips and associated parts for samples to quickly enter flow cytometers. Syringe-based systems also require cleaning of the syringe to reduce contaminating cells. Follow user manuals and log maintenance to maintain data integrity.
Comparing technical specification documents
- Purpose of technical specification sheets
- Comparing specifications from multiple manufacturers
- Pay special attention to: laser power
- Pay special attention to: pinholes and laser alignment design
- Pay special attention to: maximum event rate of theoretical maximum event rate
- Pay special attention to: carryover
- Pay special attention to: operating temperature
- Pay special attention to: size and weight
- Pay special attention to: plate analysis speed
- Conclusion
Purpose of technical specification sheets
Flow cytometer manufacturers provide technical specification sheets (tech specs or spec sheets) that describe the instruments’ key performance characteristics, and these documents can contain a wealth of information for those interested in purchasing a flow cytometer. Comparing various tech specs, however, can be challenging because their values may have been calculated in different ways, despite sharing the same terminology.
The purpose of the spec sheet is to help you identify design attributes of the flow cytometer, such as performance, size, environment, and software, to determine if the instrument is a good fit for your research.
Comparing specifications from multiple manufacturers
Technical specifications can be used as a basis for comparison, helping you assess the value of different instruments for the price. The spec sheet is also a guide to the performance that the manufacturer will warrant. For this reason, you should have a good understanding of the stated values and how they pertain to your intended use of the instrument. When using the spec sheet as a comparison guide across platforms, be inquisitive. There are many performance values that appear comparable across instruments but in reality are quite different. A specification is derived from a specific test or calculation, but these tests are not standardized across instrument developers and may be misleading in a side-by-side comparison.
Comparing various tech specs can be challenging because their values may have been calculated in different ways, despite sharing the same terminology. This white paper provides helpful hints about how to decipher the variations.
Sections within a technical specification sheet vary from one manufacturer to another, adding additional variability. Commonly published categories of information are described in Figures 1–8 using the spec sheet for the Invitrogen Attune NxT Flow Cytometer as an example. Key specifications that require special attention during flow cytometer evaluations are discussed, including helpful hints about how to decipher the variations.
Figure 1. Fluidics. The fluidics system of a flow cytometer transports the sample from the sample tube to the flow cell. Once through the flow cell (and past the laser and detector), the sample is transported to waste. The fluidics section in a spec sheet provides information about the sample, volume, flow rates, flow cell, and fluidic capacity.
Figure 2. Optics. As an analysis platform, flow cytometry relies on interrogation of individual cells by laser light and the collection of the resulting fluorescence and scatter. The optics system handles illumination and light collection within the instrument. The optics section in the sheet provides overviews of optical specifications such as laser type and power, laser profile, alignment, and photomultiplier tubes (PMTs).
Pay special attention to: laser power
Specified in mW
The power specification is most frequently defined as the laser’s output as published by the laser manufacturer (Figure 2). However, the power specification stated does not necessarily correspond with the power that is actually delivered at the point of interrogation, because light losses in the optical components between the laser and the flow cell can be large. This light loss varies from system to system. High light loss means that some of the laser’s power is not being fully utilized in the experiment; reduced light loss means higher laser intensity on the flow cell, leading to greater excitement of the fluorophores and greater sensitivity. Because of this variability, a higher number for laser power indicated on the spec sheet does not mean that the instrument is more sensitive than another.
How to compare
Be cautious in how much you rely on this specification when comparing instruments. Most manufacturers don’t report the actual power that is reaching the flow cell, but this is where the comparison should be made.
Pay special attention to: pinholes and laser alignment design
This specification, which is not reported by every manufacturer, refers to the number of pinholes that determine if the fluorescence signal generated by each laser is separated at the detectors (also called PMTs). You should know if the system’s lasers are spatially separated by internal pinholes or whether the system is collinear. Pinholes allow for maximal excitation of fluorophores and minimal crosstalk between the laser lines. Several flow cytometer manufacturers utilize collinear lasers. When lasers are aligned in a collinear fashion through a single pinhole, there is a collection of signal from more than one laser in the same optical path. This configuration means that the response of several dyes excited by different lasers is measured by the same detector, which can affect compensation values and lead to difficulty in analysis. Other risks in this design include susceptibility to alignment issues (increased coincidence rate if the beams are not exactly collinear) or reduced sensitivity for dim labels. An alternate design is a spatially separated system (Figure 2). This configuration has several benefits, including resistance to alignment problems, more choices for laser colors, and improved compensation for multicolor panels.
How to compare
Be sure you have a clear understanding of the type and quantity of lasers that are assigned to each pinhole, and ask if the system under consideration uses spatially separated or collinear lasers.
Figure 3. Performance. Of the typical specifications published widely among manufacturers, the performance section of a spec sheet contains several common features, such as MESF calculations, data acquisition rate, and parameters for forward scatter (FSC) and side scatter (SSC).
Pay special attention to: maximum event rate or theoretical maximum event rate
Specified as events/sec
The event rate is the physical count of the cells or particles as they pass through the instrument’s interrogation point. Two basic yet quite different methods are used to determine what the value is, even though this value is generally cited in the same way (i.e., events/sec). The two methods for calculating event rate are:
- Assessing how fast the electronics can process events
- Assessing the maximum event rate once a specific coincidence rate has been reached
It’s important to understand how the manufacturer arrived at this value in the spec sheet.
Method 1
The first method is theoretical and disregards the rate of coincidence and other instrument design features such as the system fluidics. Thus, while the electronics may be able to process events at 10,000–100,000 events/sec, this event rate may correspond to a coincidence rate well above the generally accepted 10% rate limit according to Poisson distribution. This way of presenting the specification for event rate has become more popular in recent years due to the advent of faster (though not necessarily higher-quality) electronic circuitry; however, event rates calculated this way don’t take coincidence into consideration.
Method 2
The second method of determining an instrument’s event rate is based on a 10% level of coincidence, which is more relevant to researchers (Figure 3). Lower coincidence rates indicate higher data integrity. Therefore, using this method best represents acceptable coincidence at an actual event rate that can be used to generate high-quality data. Users can be more confident that the data will be within acceptable coincidence rate levels at a given event rate when manufacturers report their specification for event rate using this method.
How to compare
When deciding between instruments, be sure that you know how the manufacturer arrived at the specification for event rate. If this value was calculated using the first methodology (speed of electronics only), you should carefully examine the coincidence rates when running your samples.
Pay special attention to: carryover
Carryover refers to the amount of an original sample that carries over and contaminates the next sample, resulting in data inaccuracies (Figure 3). The percent carryover specification is usually measured by acquiring a fixed volume of sample, followed by acquiring a fixed volume of a particle-free, buffer-only solution such as phosphate-buffered saline (PBS). Events representing the contaminating cells are then identified in the buffer only solution. Many manufacturers run a specific cell line or set of beads to determine this carryover value under defined conditions.
How to compare
It’s important to find out what these defined conditions are and what sample was used to determine a carryover value. For example, the number of washes or the size or type of particles or cells used for the specification test may be vastly different from what a researcher would use. Ask the manufacturer how they arrived at their stated carryover value. Knowing how different manufacturers calculate this value can help you make a direct comparison of carryover just by referring to the specification.
Figure 4. Software. The software section of a spec sheet enables researchers to identify key capabilities and features built into the system. This section also indicates the extent of functionality, user-definable features, maintenance features, and user account administration. Software features vary among manufacturers and some systems have unique, exclusive options.
Figure 5. Quality and regulatory. Quality and regulatory specifications indicate manufacturing integrity, warranty, field engineering procedures, and ISO credentials.
Figure 6. Data management. This section is key for successful computer installation and setup. Specifications within this section detail RAM, hard drives, and FCS format.
Pay special attention to: operating temperature
Specified in °C or °F
This specification is often overlooked, yet can be important to the lifetime value of your instrument because optical alignment and fluidics are highly coupled to these values (Figure 7).
How to compare
Determine if the lab remains at relatively constant temperature and if the instrument will be used in a variety of places. Instrument performance is tested and warranted only within the specified temperature range, so be sure to consider the conditions of operation.
Figure 7. Installation requirements. A typical requirements section denotes information about the environmental impact of the instrument, operating conditions, and footprint.
Pay special attention to: size and weight
Specified as H x W x D in cm or in.; instrument weight in kg or lb
Consider whether the instrument will fit in the desired space or area available in a fume hood. Be sure to include the space requirements for accessories such as an autosampler and find out if any space will be necessary for the fluidics (Figure 7). An external fluidics system is common for many flow cytometers and can add substantially to the overall space needed for the instrument. If the instrument will be moved periodically, think about how difficult this might be. Be aware that not all benchtop instruments have the same space requirements or portability.
How to compare
When a demo is being performed, request to see the full system with all components set up so that you’re able to directly compare the space needed for each system.
Pay special attention to: plate analysis speed
Specified in minutes per 96- or 384-well plate
Plate-speed values are typically defined as the time required to complete analysis of a 96- or 384-well plate (Figure 8). There are two aspects to this definition: one is the sample volume, the other is the sample-processing rate. Be aware that the time value reported in this specification may represent a sacrifice in data quality. Also, be sure to get clarity on what volume of sample was analyzed and what sample processing rate was used to determine the value in this specification. Some manufacturers choose to show a specification figure that minimizes plate times by collecting low-volume samples. However, in practice this could require samples to be highly concentrated, resulting in data quality issues like higher coincidence and abort rates. Conversely, if samples aren’t concentrated enough, a low volume may lead to a lack of enough events to be statistically significant.
The sample-processing rate (the rate at which the sample is being introduced to the fluidics system) can also affect the data quality. In flow cytometers that rely solely on hydrodynamic focusing, the sample is spread across a wider core stream as the flow rate increases. Higher sample rates produce greater variability, less precise measurements, and compromised data quality. With instruments that utilize acoustics-assisted hydrodynamic focusing, the cells remain tightly aligned in the center of the stream regardless of the sample rate, resulting in less signal variation and improved data quality. Therefore, if you choose to increase the sample input rate in order to lower plate times, you should use a system that offers acoustic focusing to avoid loss of data quality.
Another variable in calculating the plate analysis speed is whether the probe is rinsed between samples. Probes that are not rinsed introduce the potential for higher carryover—a tradeoff that should be considered before making a decision to run experiments at the given specification for plate analysis time.
How to compare
Make sure you understand the tradeoff in data quality that may be incurred to achieve the times the manufacturer represents with this specification.
Figure 8. Sampler specifications. A sampling device is an accessory to the flow cytometer. Specifications of this device include acquisition time, carryover, and mixing method. Additional information regarding compatible plate types and fluidics options may also be included.
Conclusion
Always inquire about the tests associated with pertinent specification values, so you can be confident that you’re making accurate comparisons between the features of the instruments under consideration.
Evaluating service and support prior to purchase: the importance of considering the aftermarket capacity and level of excellence of each manufacturer
- Post-purchase instrument care: a relationship, not a transaction
- Five qualities to look for when choosing a flow cytometry company
- Criteria for choosing a flow cytometry company
- High-performance service and support teams
- Think beyond the instrument
- Critical Consideration: Repair
- Critical Consideration: Training
Post-purchase instrument care: a relationship, not a transaction
When you buy an instrument, you are buying the manufacturer behind that instrument and need to consider the willingness, infrastructure and scalability of the manufacturer’s ability to provide you with the adequate long-term, after-sales service. This is not only a transaction, but a relationship between you and the manufacturer’s team. The extent and dynamics of this relationship vary depending on what you need and require from the team.
Carefully consider how each candidate manufacturer is able to provide you with each aspect of the proper care and feeding of your flow cytometer.
Five qualities to look for when choosing a flow cytometry company
Investigate a company’s capacity and commitment for servitization. Look for an ambidextrous, product-and-service–focused organization that seeks and respects feedback and actively incorporates improvements to products and service.
- Active feedback loop with install base—incorporates listening that connects all the way back to engineering and R&D to produce incremental innovations and NPI stemming from customer feedback
- Portfolio availability—comprehensiveness of instrument plus reagent portfolio
- Tenacity—improvement, innovation, and functionality
- Humility—a company not taking for granted that researchers will buy their instrument
- Proximity to the biology—beyond mechanical in each function, cognizant of the projects the instrument owners will be undertaking
Criteria for choosing a flow cytometry company
A capable flow cytometry company will have a diverse team of people working multiple functions to support their customers. Ensure the flow cytometry company you do business with offers:
- Multiple, complementary and interdependent functional teams beyond service engineers
- A global Infrastructure with the resources and capability to service each customer as if they were their only customer
- A team of people—the most impactful, sustainable aftermarket care program of a manufacturer is a customer-centric, resource-rich team
High-performance service and support teams
- Field Service Engineers—the front line of installation and repair
- Engineers—creative software and mechanical systems experts devising better ways to do flow cytometry
- R&D—bench scientists developing and validating high-performance antibodies and reagents
- Application Scientists—high-aptitude research scientists with proficiencies across a diverse palette of techniques and applications
- Technical Assistance Group—problem-solving, tribal-knowledge keepers who interact with
- Logistics—masters of getting everything from A to B to you
- Product Managers—orchestrating portfolio development to expand options for customers
- Technical Sales Specialists—partners who identify and customize best-fitting solutions and configurations now and over time
- Market Development & Customer Education—content managers who curate volumes of learning resources
- Executive Leadership—team builders who lead the team to goal achievement and authentic camaraderie
Think beyond the instrument
Ask: How comprehensive is the portfolio?
Why you should ask: Multiple advantages are associated with narrowing the diversity of brands and manufacturers from which you order to manage your lab, including:
- Orchestration—increased instrument, component, and software compatibility for faster set up and implementation
- Operation—convenience and advantageous pricing in ordering and service
- Orientation—increased consistency across staff and operator training, protocol, systems, and maintenance
Critical Consideration: Repair
Breakdown, glitches, and irretrievable clogs can require repair, creating a standstill and costing stalled output, lost samples, wasted sample prep time, and repeated assays. Selecting a flow cytometer with quality engineering, craftsmanship, and hardware (e.g., high-end pumps and lasers), can help reduce the frequency of needed repairs and boost the instrument’s performance and lifespan. Additionally, a competent service and support team can shorten the time to recover and reinstate operation when there is a problem requiring repair.
Characteristics of a strong service-focused team
- Sense of urgency—Responsiveness of service is critical, especially in environments already subject to unpredictable timing and long runtimes.
- Retention of a strong technical competency base—Bench-experienced, credentialed field service engineers, technical assistance partners, complex application specialists, and training officers
- Efficient execution and escalation—Swift delivery of all components, consistency in interactions and protocol
Repair discussion questions
- Along with response time, how long on average are repair tickets open—especially in cases where multiple attempts are made to complete the repair? What is your average speed to resolve?
- Are repair parts new?
- Are repair parts manufactured by the same vendors that supply the parts for the initial unit?
- Are you able to remotely log into our system using internal remote performance sensors and help us troubleshoot?
- Are repairs handled by outside contractors, or are all of your field service engineers employees?
- Do you offer timeframe guarantees for the dispatch of a field service engineer?
- How many different types of instruments are your field service engineers responsible for understanding? Are any dedicated completely to flow cytometers?
- How often are your repair engineers retrained on this specific type of instrument?
- If a field service engineer replaces a part but the instrument still doesn’t work, is our open ticket escalated?
- If a ticket is escalated, then who shows up to fix the instrument?
- If the instrument goes down, will you send a replacement or a loaner? When?
- What extended repair hours do you offer? Will my middle-of-the-night call be routed to middle-of-the-day tech support staff in another time zone?
- What is the territory size, availability, and experience level of the field service engineer who will be assigned to our account?
- Will we have a dedicated repair technician?
Professional maintenance
- How extensive is the scheduled routine professional maintenance?
- How often is maintenance needed?
- Are any routine visits from a field service engineer included in the purchase?
Critical Consideration: Training
With any technology, you have to know how to use it correctly. Flow cytometry can be challenging at first, but the basics are used across all platforms. As new users learn about panel design, compensation, fluorophore selection, and other core skills, they can progressively increase the complexity of their experiments. When selecting an instrument, find out what in-person training, online educational materials, and user communities are available through the manufacturer.
Characteristics of a strong, service-focused team
- Fully-operative customer onboarding program
- Plentiful and generous educational and training resources
- Quality, academically-credentialed trainers
Usability is a relative term, and correlates to the extent of a person’s knowledge of and experience with flow technique, the instrument’s software UX design, the user’s technological aptitude, the relationship between the limitations of their detection equipment and their biology, and the objectives of their experiments. A training program should address these nuances and be able to service beginners, as well as competently laisse with advanced users.
Training discussion questions
- Do you hold user meetings?
- Do you offer active, hands-on training for topics such as panel design and compensation?
- Do you offer additional, comprehensive training? How much does it cost?
- Following the training, will we receive a training manual to keep? If so, how comprehensive is the material?
- Where and how soon after installation will training be scheduled?
- Is the initial training in-person or online?
- If training happens in a facility, will we be learning on the same version of software that comes with the instrument?
- Is ongoing training offered as we get new users?
- What level of complexity is the offered curriculum: beginning, intermediate, or advanced?
- Who is responsible for training new users at a user training center or in their own labs?
- Who trains the trainer at your company?
Decision Workbook: Choosing an extraordinary flow cytometer
This digital workbook features the experimental steps to compare instruments from different manufacturers head to head and a model for how to approach group decision-making dynamics and group accountability in order to reach a consensus supported by measurements plus dialogue.

Fluidics hardware in a flow cytometer
Flow cytometry interrogates single cells or other particles suspended in fluid for multiparametric analysis of properties. The flow of cells or particles gives a distinct advantage over other methods such as imaging and PCR by collecting information from single cells in a sample. Sophisticated technology is required for a sample to enter the instrument and be delivered to the optical system.
The importance of having a good fluidics system

The power of flow cytometry is the ability to quantitate the physical characteristics of individual cells in a heterogeneous population. This process requires the fluidics system to deliver large numbers of cells into the flow cytometer in order to produce a statistically significant amount of data. The flow of cells into and through the instrument must be consistent for accurate counting and measurements.
Coincidence in high-speed flow cytometry

Figure 1. Coincidence rate. (A) One cell is excited by the laser beam. (B) Two cells are excited by the laser beam producing a doublet signal. Cells in doublets and triplets can mask the true biological nature of a sample.
Cells enter into the flow cytometer in an unaligned, arbitrary fashion. Cells ideally flow past the interrogation laser in single file for individual detection, but there are some instances where the cells flow through together (as governed by Poisson distribution or samples with cell clumps). When there is more than one cell in the laser path at the same time, this is called coincidence.
Two cells simultaneously passing through the laser can lead to difficulty in distinguishing the cells of interest. If one cell is positive and the other is negative, and they both pass together through the laser, the instrument will read the event as a positive signal. When this occurs, the data obtained from this event is a doublet event and not usable. The technology behind the focusing system provides the mechanism for single-cell interrogation in the optical system.
Fluidics delivery systems

Figure 2. Systems for sample entry into the flow cytometer. (A) Pressure-based fluidics rely on the pressure difference between the sample fluid stream and the sheath fluid surrounding the sample stream. (B) Volumetric-based fluidics provide the ability to obtain a concentration and count measurement since the exact sample volume can be measured.
Pressure-based and volumetric-based fluidics are two common mechanisms for cells to enter into the flow cytometer.
Samples with large volumes of cells can quickly enter the instrument with pressure-based fluidics. A pressurized system is created by a specialized tube forming a seal with the instrument. The sealed tube initiates a difference between the pressure of the sample and of the instrument sheath fluid to move cells into the optical system. Pressure-based systems allow for smooth sample delivery, larger sample volume, and less risk of running out of sample. Backpressure is a problem with this type of system, and it can clog if it is not properly maintained.
Volumetric-based fluidics enable more flexible cell delivery. The mechanism for this system measures a precise volume of sample and injects it into the instrument. Benefits include the ability to determine absolute cell count and accommodate difficult samples, including sticky cells or large cells, by being less sensitive to clogging from backpressure. This system has added value as any type of tube or plate can hold sample and deliver it into the system.
Sample delivery pumps

Figure 3. Two types of pumps. (A) Peristaltic pumps move fluids using a rotating-head system. The rollers in the head depress the tubing and drive the fluid forward. (B) The syringe pump draws up a sample and injects it into the fluidics system.
Sample delivery in pressure- and volumetric-based systems is accomplished by using either peristaltic or syringe pumps.
Peristaltic pumps are a common, low-cost alternative for less-expensive instruments. Fluidics systems equipped with these pumps employ a series of rotating rollers to press on the tubing. This type of system can quickly move large sample volumes. This type of technology, however, does not provide accurate cell count as the amount of sample delivered into the instrument fluctuates with the rotation of the pump.
Volumetric delivery systems rely on a syringe pump in order to precisely control and measure sample volume. Syringe pumps provide the mechanism for absolute cell counting and sample recovery. Returned sample can be used for other experiments such as PCR and imaging or stored to run again. Volumetric sample delivery represents a quality, well-crafted designed for precise delivery of cells into an instrument.
Technology to flow suspended cells into the optics system

Figure 4. Cell focusing systems.
Single cells flowing through the optical system can be obtained using different methods of fluidics in an instrument.
Most flow cytometers use traditional fluidic focusing technology such as hydrodynamic focusing. This technology uses the difference in pressure and speed between the sheath fluid and cell sample to move a cell forward.
Other fluidics systems use acoustic-assisted hydrodynamic focusing. This focusing method is a modification of the traditional system and uses sound waves to focus cells into a middle of the flow cell and traditional hydrodynamics to deliver the cells to the optical system. Acoustic-assisted hydrodynamic focusing reduces fluid usage while maintaining the benefits of hydrodynamic focusing. The combined use of acoustic focusing and sheath fluid aligns cells into a single file, resulting in some experiments running at quicker speeds with lower coincidence rates. The small amount of sheath fluid has an added benefit of keeping the flow cell clean and less prone to clogging. This type of technology can flow dilute samples quickly.
Sample handling
Pressure-based systems use tubes and mostly process one sample at a time. For high-throughput applications, these flow cytometers can be equipped with a separate multiwell sampler. Multiwell plate sampling may not be available in some models as specialized tubes are required to create the pressure differential for samples to enter into the flow cytometer.
Some volumetric-based flow cytometers have the capability to sample from both tubes and multiwell plates. The pipette-like syringe both mixes and injects the sample into the instrument.
Plate samplers can be attached to robotic handlers for high-throughput processing of many multiwell plates. These systems handle large volumes of samples by having greater fluidic support to continuously run multiple plates.

Optics options for a flow cytometer
The capabilities of an optics system in a flow cytometer will directly influence how an experiment is designed and executed. Lasers provide the light to excite fluorophores conjugated to antibodies or reagents for use in the detection and analysis of various components and aspects of the cell. Detectors are the devices that capture the light of specific wavelengths, amplify, and resolve this light to provide the information of the specific physical characteristic details the fluorophore is meant to measure. The technology behind the lasers and detectors dictates the number and type of fluorophores used in an experiment. This is why it’s important to understand the options of the many components that create the optical system in order to obtain optimal data.
The importance of having a good optics system

The identification of a cell and its physical characteristics is dependent on the power of the laser beam and the sensitivity of the detection system. Most experiments require multiple fluorophores to tag cell surface and intracellular proteins in order to identify cell populations, cell cycle, population doublings, apoptosis, and cell viability. Good optics systems with quality components will capture the full-range of biologic and biochemical properties of a cell.
An optical system with sensitivity and range is important as fluorophores emit light in a color spectrum
Laser excitation and emission wavelengths of fluorescent dyes

Figure 5. Laser excitation and emission wavelengths of fluorescent dyes. (A) Cell with multiple fluorescent labels. (B) PE and FITC are both excited by the blue 488 nm laser and have overlapping emission spectrums. (C) PE is excited by the yellow 561 nm laser, but FITC is not. (D) Alexa Fluor 647 dye is excited by the red 640 nm laser.
Each fluorophore has an excitation energy range and a specific light-emitting spectrum. Cells can be labeled with multiple fluorescence-labeled antibodies, fluorescent dyes, and fluorescent proteins. Lasers emit power at specific wavelengths to provide the different amounts of required excitation energy.
Spectral overlap (fluorescence overlap)

Figure 6. Double excitation maximums. (A) PE can be excited with the 488 nm laser. (B) PE has another excitation maximum at 561 nm. Excitation of PE by this laser more efficiently emits fluorescence.
Some fluorophores have multiple excitation wavelengths of different efficiencies. For example, either a blue 488 or yellow 561 nm laser can excite the fluorophore PE. A blue 488 laser excites PE at a lower energy level. A yellow 561 nm laser provides more excitation to the fluorophore and results in a greater amount of emitted photons. Stronger emitted light may resolve a small, dim population of cells that might not have been seen on an instrument without a yellow 561 nm laser.
Optics component technology
Lasers

Figure 7. Laser wavelengths with common fluorescent proteins and select dyes.
Commercially available flow cytometers may include violet (405 nm), blue (488 nm), green (532 nm), yellow (561 nm), and red (640 nm) lasers. Base instruments typically include the blue 488 nm laser. Adding more lasers provides the option to increase the number of fluorophores that may be used in one experiment. Violet, blue, yellow-green, and red lasers are sufficient to excite most fluorophores used in multi-fluorophore experiments. Some flow cytometers can be equipped with a UV laser in the 350 nm range to spread out the excitation and emission wavelengths when designing multicolor experiments.
Laser profile

Figure 8. Laser alignment. (A) Cells receive full laser power with properly aligned lasers. This ensures each cell will have similar energy for fluorophore excitation, providing less data variation. (B) Laser misalignment may cause uneven distribution of energy for fluorophore excitation. This may increase the chances for data variation.
Every cell should receive similar laser excitation energy in order to compare emitted fluorescent signals between cells in an experiment. Consistent laser beam output with correct laser alignment helps provide an optimal signal to every cell that passes through the interrogation point. Well-aligned laser beams result in data produced by biological, not instrument, variation.
In samples with weakly labeled cells, suboptimal excitation energy can lead to negative results. When laser power is not consistent, there will be more variation between collected emissions from cells resulting in greater statistical error. Shifts in laser alignment may provide less or unequal excitation energy to each cell passing through the beam.

Figure 9. Laser profiles. (A) Gaussian emission profile. Photons are dense and most intense in the center. (B) Flat-top beam intensity profile covers a larger and more flat area. Cells pass through a wider apex to generate fluorescence.
Gaussian laser beams are found in most flow cytometers. Cells must pass through the center of the beam for optimal excitation energy.
Recently developed flat-top laser profiles cover more area to excite fluorophores. The wider apex allows cells in a sample to have an even application of energy to help produce consistent results.
Laser arrangement

Figure 10. Laser arrangement. (A) Collinear laser arrangements have multiple lasers arranged to focus on a single spot of the interrogation point. (B) Spatially separated lasers are individually arranged and focused along the interrogation point.
Multiple lasers in one instrument are most commonly arranged in two formations. The arrangement affects both the number of fluorophores used in an experiment and also the detection of cell fluorescence.
Collinear spatial arrangement fires all lasers at the same time to focus laser light onto one area, resulting in the simultaneous excitation of many different fluorescent dyes.
This laser arrangement is less flexible in multicolor panel building as multiple fluorescence signals are simultaneously emitted and require greater compensation. A collinear laser arrangement works well with lasers of two different wavelengths that excite fluorescent dyes with minimally or non-overlapping emission spectra. Less expensive flow cytometer instruments may have this type of laser arrangement to save on component cost.
Spatially separated arrangement sequentially fires lasers on the cell stream. Cells stained with multiple fluorophores pass through each laser beam one at a time for separate excitation and detection of emission spectra. This type of arrangement provides flexibility for the number of dyes used in one experiment. The individually emitted fluorescence signals provides higher sensitivity and requires less compensation. Large, multicolor panels can be more readily built using this arrangement as it generates significantly less fluorescence overlap in similar emission spectra.
Fluorescence separation components

Figure 11. Fluorescence emission. (A) Excited fluorophores emit light in a limited range of wavelengths. (B) Some fluorophores can have overlapping emission spectra.
Excited fluorophores emit light in a limited spectrum, not at a specific wavelength. Emitted fluorescence needs to be passed through optical filters before it is captured and analyzed. Without fluorescence separation, the unfiltered light from multiple fluorophores would overlap and be difficult to analyze

Figure 12. Components for fluorescence separation. (A) Optical filters divide the emitted signal from fluorophores based on the fluorescence wavelength range. (B) Prism monochromator arrays separate light into regions of different wavelength and focus light from these regions onto different detectors.
Cells labeled with multiple fluorophore-conjugated antibodies can be analyzed because of the development of optical filters. These light separation components are the most commonly used for fluorescence separation in flow cytometers as they are easily accessible and user interchangeable. Combining different filters allows for varying color combinations to create both small and large multicolor panels. This is a tried-and-true system with durable components. Look for a system with easily changeable filters.
Spectral flow cytometry is different from conventional flow cytometry. Instruments with this technology use prisms to focus emitted fluorescence in the visible light spectrum. Light emitted from multiple fluorophores will overlap and requires a second step with complex algorithms for separation. The benefit of this technology is that it expands the total number of color channels from fewer lasers. This technology is still developing for flow cytometry, and computational fluorescence separation maybe limited for certain fluorophores.
Emission detectors

Figure 13. Components for emission detection. (A) PMTs capture and multiply signal emitted by a fluorophore. (B) APDs capture photons on a semiconductor and then multiply the signal.
The emitted fluorescence from a single cell is a weak signal. The light needs to be converted into an adjustable form of data for analysis. An emission detection device captures fluorescence light and converts the light waves into an electronic signal. This step is important as individual electronic signals can be amplified and the data can then be processed by the user.
Photomultiplier tubes (PMTs) are detection devices found in the majority of flow cytometers. Multiple PMTs are arranged at the end of the optical path and capture each filtered fluorescence signal. This type of detection device is useful with multicolor panel experiments because PMTs can capture a dynamic range of fluorescence, from very dim to very bright signals in a linear manner from the broad range of the spectrum from UV to IR.
Alternative detection devices include the avalanche photodiode (APD) to conduct and multiply electrons generated from emitted fluorescence. Experiments that heavily use fluorophores emitting in the far-red spectra can benefit from APDs. These detectors efficiently capture signals emitted in the red and near- infrared spectral regions.
Voltage settings

Figure 14. Voltage settings. (A) Positive signal emitted from a fluorophore-labeled cell is not in the detectable range and is off scale because the PMT voltage is too high. (B) The voltage is lowered and the positive signal is now detectable.
Sample background may be detected as autofluorescence from cells or other components of a sample running on a flow cytometer. Fully visualizing the physical characteristics of stained cells requires capturing the full range of negative (dim) signal through positive signal. In other words, the ideal PMT voltage must be adjusted to view the maximum separation between the associated stained fluorophore and the cell’s detectable (negative) autofluorescence.
Before running cell samples, PMT voltages of the flow cytometer should be optimized using a fluorescent standard to calculate the optimal voltages for each PMT.
A PMT voltage may be adjusted if necessary, especially if the positive signal is too bright. Users may adjust the voltage by using unstained control cells as a negative for background signal. Lowering the voltage of the PMT allows proper measurement of the positive signal and decreases the intensity value of the negative population.
The amount of signal from fluorescence light detected by the flow cytometer is controlled by voltage settings. Setting a higher or lower voltage produces more or less gain on the PMT detector for proper fluorescence signal measurement.
Locked voltage settings are part of simple flow cytometer function, thus simple flow cytometers offer a limited range of detectable emitted fluorescence. Adjusting voltage settings can be challenging since the user needs to be able to distinguish positive cell populations from background. New users may over- or under-adjust voltages. To help these users, voltages are locked for a small panel of fluorescent dyes and their associated detectors. Instruments with locked settings simplify the process by limiting the ability to change voltage settings.
Unlocked or adjustable voltage settings are preferable for users running multicolor panel experiments. Locked voltage settings are preset to detect fluorescence emitted from a limited panel of fluorescent dyes. Adjustable voltage settings are not limited to a set panel of fluorescent dyes. This feature allows users to employ larger panels or include more types of dyes. Adjustable voltage settings also allow users to check their positive, single-stained controls to place their PMT signals on scale. PMT voltages may also be adjusted for optimal signal compensation as part of multiparameter flow cytometry experiments.
Fluorescence compensation

Figure 15. Compensation. (A) Overlapping emitted signals can produce false-positive data points. (B) Compensating data results in the removal of the overlapping emitted fluorescence. The compensated data points will produce more accurate results.
Emission from multiple fluorophores produces a range of light in the visible spectrum. Data collection from multicolor panels will produce overlapping fluorescence signals. This can be problematic as positive single-labeled cells may appear as double-positive cells.

Figure 16. Compensation techniques. (A) Traditional compensation uses well-researched sets of algorithms analyzing data from fluorophores, negative gating, or unstained controls to process overlapping emission into a single color. (B) Spectral unmixing uses sets of algorithms such as constrained Poisson regression or nonnegative matrix factorization to process light into a single color.
Compensation is the separation of signal from each fluorophore by a set of algorithms. Traditional compensation separates specific wavelengths. The process measures each fluorophore individually and then uses algorithms to create a matrix of correction values. These corrections are applied to the spectral overlap to produce individual values. This process straightforwardly and quickly computes signal compensation.
Spectral unmixing is different from traditional compensation in that it provides the spectrum of emitting light versus a single wavelength.
Channels and the number of fluorophores in an experiment
Figure 17. Channels. (A) A one-laser system can have multiple channels. (B) The emitted light overlaps and requires compensation. (C) A 3-laser system will have more channels because there are more detectors. (D) The channels are spread out, allowing for more fluorophore options and less compensation.
Components to capture emitted light in both PMTs and APDs are called channels. The number of fluorophores detected by a flow cytometer will be determined by the number of available channels.
A one-laser system can have multiple channels. This means a single laser can excite multiple fluorophores, and the detectors can capture emitted light at different wavelengths from a sample. This type of experiment requires complex compensation as all fluorophores can emit light in overlapping wavelengths.
Multiple lasers may be of benefit since they provide more channels. Additional channels confer easier panel design as there are more fluorophore choices and less compensation. This may allow for an experiment with better resolution of populations.

Instrument maintenance
Flow cytometers are sophisticated instruments designed to last many years. Most components are permanent fixtures. Periodic maintenance and routine cleaning will minimize parts replacement and help provide high-quality data.
The importance of instrument cleaning and maintenance

Figure 18. Common contaminants from improper maintenance. (A) Carryover is the contamination of cells or particles from previous samples. (B) Dyes binding to nucleic acid are sticky and can create background noise. (C) Microbes can grow in fluidic bottles and other parts of a flow cytometer.
One type of problem that arises when regular instrument cleaning is not performed cleaning is the accumulation of material from previous samples.
Carryover cells from previous samples are a problem for sensitive experiments such as measurement of rare cells. The rate of false positives increases when carryover cells are present. Flushing between samples or after experiments can reduce the number of cells or particles leftover from a previous experiment.
Regular cleaning prevents buildup of fluorescent dyes; certain dyes such as nucleic acid dyes are more prone to build up. Collections of sticky dyes, cells, and debris create more opportunity for tubes to be clogged. Instruments that run cell culture or tissue samples have a higher chance of bacterial contamination. Signs of contamination include cloudy fluid in the waste bottle and a high number of event collections in blank samples. Running cleaning solution or bleach through the system according to the manufacturer’s instructions can discourage microbe growth.
Routine cleaning

Figure 19. Daily cleaning. (A) Run bleach and DI water between samples. Some instruments can automatically clean. (B) Startup and daily shutdown cleaning procedures help prevent buildup of dyes and carryover of cells. (C) Additional daily maintenance checks may involve automated protocols with software guidance.
Minimize contamination between samples with cleaning. Between each user experiment, most manufacturers advise users to run 10% bleach then DI water before running the next experiment. Some instruments have automatic cleaning between samples.
Most systems require distilled water and cleaning solution before use and at the end of the day to remove cells and dyes from flow cytometer tubes and flow cells. Startup and shutdown cleaning protocols are detailed in most user manuals. Daily maintenance helps ensure fluidic tubes and flow cells are free from contamination with microbes or old sample.
Some flow cytometers have additional daily maintenance requirements including fluid tank preparation, a check for air bubbles trapped in filters, and emptying waste containers. Other flow cytometers have automated protocols with software guidance. Automated systems reduce user responsibility by removing certain tasks such as air bubble checks or shutdown procedures. These protocols are in place to save time and provide accurate cleaning.
Fluidics decontamination

Figure 20. Fluidics maintenance. (A–F) Clean or replace flow cytometry instrument parts to maintain normal instrument performance and minimize carryover, background noise, and microbial contamination.
A combination of fluidics flushing, changing of sheath filters, and deep instrument cleaning can remove carryover, fluorescent dyes, and microbes. Most flow cytometers require a deep cleaning of sheath tubing, waste tubing, fluidic containers, and the flow cell every 2 weeks to one month. This prevents the buildup of debris and removes biohazardous material and microbes.
Depending on the type of fluidic system, components may have to be removed and replaced or cleaned . Peristaltic systems require that tubing be removed and replaced every 6 months to a year. Without replacement, contaminants or debris will appear during event collection. Pressure systems require regular maintenance of nozzle tips and associated parts for samples to quickly enter flow cytometers. Syringe-based systems also require cleaning of the syringe to reduce contaminating cells. Follow user manuals and log maintenance to maintain data integrity.
Comparing technical specification documents
- Purpose of technical specification sheets
- Comparing specifications from multiple manufacturers
- Pay special attention to: laser power
- Pay special attention to: pinholes and laser alignment design
- Pay special attention to: maximum event rate of theoretical maximum event rate
- Pay special attention to: carryover
- Pay special attention to: operating temperature
- Pay special attention to: size and weight
- Pay special attention to: plate analysis speed
- Conclusion
Purpose of technical specification sheets
Flow cytometer manufacturers provide technical specification sheets (tech specs or spec sheets) that describe the instruments’ key performance characteristics, and these documents can contain a wealth of information for those interested in purchasing a flow cytometer. Comparing various tech specs, however, can be challenging because their values may have been calculated in different ways, despite sharing the same terminology.
The purpose of the spec sheet is to help you identify design attributes of the flow cytometer, such as performance, size, environment, and software, to determine if the instrument is a good fit for your research.
Comparing specifications from multiple manufacturers
Technical specifications can be used as a basis for comparison, helping you assess the value of different instruments for the price. The spec sheet is also a guide to the performance that the manufacturer will warrant. For this reason, you should have a good understanding of the stated values and how they pertain to your intended use of the instrument. When using the spec sheet as a comparison guide across platforms, be inquisitive. There are many performance values that appear comparable across instruments but in reality are quite different. A specification is derived from a specific test or calculation, but these tests are not standardized across instrument developers and may be misleading in a side-by-side comparison.
Comparing various tech specs can be challenging because their values may have been calculated in different ways, despite sharing the same terminology. This white paper provides helpful hints about how to decipher the variations.
Sections within a technical specification sheet vary from one manufacturer to another, adding additional variability. Commonly published categories of information are described in Figures 1–8 using the spec sheet for the Invitrogen Attune NxT Flow Cytometer as an example. Key specifications that require special attention during flow cytometer evaluations are discussed, including helpful hints about how to decipher the variations.
Figure 1. Fluidics. The fluidics system of a flow cytometer transports the sample from the sample tube to the flow cell. Once through the flow cell (and past the laser and detector), the sample is transported to waste. The fluidics section in a spec sheet provides information about the sample, volume, flow rates, flow cell, and fluidic capacity.
Figure 2. Optics. As an analysis platform, flow cytometry relies on interrogation of individual cells by laser light and the collection of the resulting fluorescence and scatter. The optics system handles illumination and light collection within the instrument. The optics section in the sheet provides overviews of optical specifications such as laser type and power, laser profile, alignment, and photomultiplier tubes (PMTs).
Pay special attention to: laser power
Specified in mW
The power specification is most frequently defined as the laser’s output as published by the laser manufacturer (Figure 2). However, the power specification stated does not necessarily correspond with the power that is actually delivered at the point of interrogation, because light losses in the optical components between the laser and the flow cell can be large. This light loss varies from system to system. High light loss means that some of the laser’s power is not being fully utilized in the experiment; reduced light loss means higher laser intensity on the flow cell, leading to greater excitement of the fluorophores and greater sensitivity. Because of this variability, a higher number for laser power indicated on the spec sheet does not mean that the instrument is more sensitive than another.
How to compare
Be cautious in how much you rely on this specification when comparing instruments. Most manufacturers don’t report the actual power that is reaching the flow cell, but this is where the comparison should be made.
Pay special attention to: pinholes and laser alignment design
This specification, which is not reported by every manufacturer, refers to the number of pinholes that determine if the fluorescence signal generated by each laser is separated at the detectors (also called PMTs). You should know if the system’s lasers are spatially separated by internal pinholes or whether the system is collinear. Pinholes allow for maximal excitation of fluorophores and minimal crosstalk between the laser lines. Several flow cytometer manufacturers utilize collinear lasers. When lasers are aligned in a collinear fashion through a single pinhole, there is a collection of signal from more than one laser in the same optical path. This configuration means that the response of several dyes excited by different lasers is measured by the same detector, which can affect compensation values and lead to difficulty in analysis. Other risks in this design include susceptibility to alignment issues (increased coincidence rate if the beams are not exactly collinear) or reduced sensitivity for dim labels. An alternate design is a spatially separated system (Figure 2). This configuration has several benefits, including resistance to alignment problems, more choices for laser colors, and improved compensation for multicolor panels.
How to compare
Be sure you have a clear understanding of the type and quantity of lasers that are assigned to each pinhole, and ask if the system under consideration uses spatially separated or collinear lasers.
Figure 3. Performance. Of the typical specifications published widely among manufacturers, the performance section of a spec sheet contains several common features, such as MESF calculations, data acquisition rate, and parameters for forward scatter (FSC) and side scatter (SSC).
Pay special attention to: maximum event rate or theoretical maximum event rate
Specified as events/sec
The event rate is the physical count of the cells or particles as they pass through the instrument’s interrogation point. Two basic yet quite different methods are used to determine what the value is, even though this value is generally cited in the same way (i.e., events/sec). The two methods for calculating event rate are:
- Assessing how fast the electronics can process events
- Assessing the maximum event rate once a specific coincidence rate has been reached
It’s important to understand how the manufacturer arrived at this value in the spec sheet.
Method 1
The first method is theoretical and disregards the rate of coincidence and other instrument design features such as the system fluidics. Thus, while the electronics may be able to process events at 10,000–100,000 events/sec, this event rate may correspond to a coincidence rate well above the generally accepted 10% rate limit according to Poisson distribution. This way of presenting the specification for event rate has become more popular in recent years due to the advent of faster (though not necessarily higher-quality) electronic circuitry; however, event rates calculated this way don’t take coincidence into consideration.
Method 2
The second method of determining an instrument’s event rate is based on a 10% level of coincidence, which is more relevant to researchers (Figure 3). Lower coincidence rates indicate higher data integrity. Therefore, using this method best represents acceptable coincidence at an actual event rate that can be used to generate high-quality data. Users can be more confident that the data will be within acceptable coincidence rate levels at a given event rate when manufacturers report their specification for event rate using this method.
How to compare
When deciding between instruments, be sure that you know how the manufacturer arrived at the specification for event rate. If this value was calculated using the first methodology (speed of electronics only), you should carefully examine the coincidence rates when running your samples.
Pay special attention to: carryover
Carryover refers to the amount of an original sample that carries over and contaminates the next sample, resulting in data inaccuracies (Figure 3). The percent carryover specification is usually measured by acquiring a fixed volume of sample, followed by acquiring a fixed volume of a particle-free, buffer-only solution such as phosphate-buffered saline (PBS). Events representing the contaminating cells are then identified in the buffer only solution. Many manufacturers run a specific cell line or set of beads to determine this carryover value under defined conditions.
How to compare
It’s important to find out what these defined conditions are and what sample was used to determine a carryover value. For example, the number of washes or the size or type of particles or cells used for the specification test may be vastly different from what a researcher would use. Ask the manufacturer how they arrived at their stated carryover value. Knowing how different manufacturers calculate this value can help you make a direct comparison of carryover just by referring to the specification.
Figure 4. Software. The software section of a spec sheet enables researchers to identify key capabilities and features built into the system. This section also indicates the extent of functionality, user-definable features, maintenance features, and user account administration. Software features vary among manufacturers and some systems have unique, exclusive options.
Figure 5. Quality and regulatory. Quality and regulatory specifications indicate manufacturing integrity, warranty, field engineering procedures, and ISO credentials.
Figure 6. Data management. This section is key for successful computer installation and setup. Specifications within this section detail RAM, hard drives, and FCS format.
Pay special attention to: operating temperature
Specified in °C or °F
This specification is often overlooked, yet can be important to the lifetime value of your instrument because optical alignment and fluidics are highly coupled to these values (Figure 7).
How to compare
Determine if the lab remains at relatively constant temperature and if the instrument will be used in a variety of places. Instrument performance is tested and warranted only within the specified temperature range, so be sure to consider the conditions of operation.
Figure 7. Installation requirements. A typical requirements section denotes information about the environmental impact of the instrument, operating conditions, and footprint.
Pay special attention to: size and weight
Specified as H x W x D in cm or in.; instrument weight in kg or lb
Consider whether the instrument will fit in the desired space or area available in a fume hood. Be sure to include the space requirements for accessories such as an autosampler and find out if any space will be necessary for the fluidics (Figure 7). An external fluidics system is common for many flow cytometers and can add substantially to the overall space needed for the instrument. If the instrument will be moved periodically, think about how difficult this might be. Be aware that not all benchtop instruments have the same space requirements or portability.
How to compare
When a demo is being performed, request to see the full system with all components set up so that you’re able to directly compare the space needed for each system.
Pay special attention to: plate analysis speed
Specified in minutes per 96- or 384-well plate
Plate-speed values are typically defined as the time required to complete analysis of a 96- or 384-well plate (Figure 8). There are two aspects to this definition: one is the sample volume, the other is the sample-processing rate. Be aware that the time value reported in this specification may represent a sacrifice in data quality. Also, be sure to get clarity on what volume of sample was analyzed and what sample processing rate was used to determine the value in this specification. Some manufacturers choose to show a specification figure that minimizes plate times by collecting low-volume samples. However, in practice this could require samples to be highly concentrated, resulting in data quality issues like higher coincidence and abort rates. Conversely, if samples aren’t concentrated enough, a low volume may lead to a lack of enough events to be statistically significant.
The sample-processing rate (the rate at which the sample is being introduced to the fluidics system) can also affect the data quality. In flow cytometers that rely solely on hydrodynamic focusing, the sample is spread across a wider core stream as the flow rate increases. Higher sample rates produce greater variability, less precise measurements, and compromised data quality. With instruments that utilize acoustics-assisted hydrodynamic focusing, the cells remain tightly aligned in the center of the stream regardless of the sample rate, resulting in less signal variation and improved data quality. Therefore, if you choose to increase the sample input rate in order to lower plate times, you should use a system that offers acoustic focusing to avoid loss of data quality.
Another variable in calculating the plate analysis speed is whether the probe is rinsed between samples. Probes that are not rinsed introduce the potential for higher carryover—a tradeoff that should be considered before making a decision to run experiments at the given specification for plate analysis time.
How to compare
Make sure you understand the tradeoff in data quality that may be incurred to achieve the times the manufacturer represents with this specification.
Figure 8. Sampler specifications. A sampling device is an accessory to the flow cytometer. Specifications of this device include acquisition time, carryover, and mixing method. Additional information regarding compatible plate types and fluidics options may also be included.
Conclusion
Always inquire about the tests associated with pertinent specification values, so you can be confident that you’re making accurate comparisons between the features of the instruments under consideration.
Evaluating service and support prior to purchase: the importance of considering the aftermarket capacity and level of excellence of each manufacturer
- Post-purchase instrument care: a relationship, not a transaction
- Five qualities to look for when choosing a flow cytometry company
- Criteria for choosing a flow cytometry company
- High-performance service and support teams
- Think beyond the instrument
- Critical Consideration: Repair
- Critical Consideration: Training
Post-purchase instrument care: a relationship, not a transaction
When you buy an instrument, you are buying the manufacturer behind that instrument and need to consider the willingness, infrastructure and scalability of the manufacturer’s ability to provide you with the adequate long-term, after-sales service. This is not only a transaction, but a relationship between you and the manufacturer’s team. The extent and dynamics of this relationship vary depending on what you need and require from the team.
Carefully consider how each candidate manufacturer is able to provide you with each aspect of the proper care and feeding of your flow cytometer.
Five qualities to look for when choosing a flow cytometry company
Investigate a company’s capacity and commitment for servitization. Look for an ambidextrous, product-and-service–focused organization that seeks and respects feedback and actively incorporates improvements to products and service.
- Active feedback loop with install base—incorporates listening that connects all the way back to engineering and R&D to produce incremental innovations and NPI stemming from customer feedback
- Portfolio availability—comprehensiveness of instrument plus reagent portfolio
- Tenacity—improvement, innovation, and functionality
- Humility—a company not taking for granted that researchers will buy their instrument
- Proximity to the biology—beyond mechanical in each function, cognizant of the projects the instrument owners will be undertaking
Criteria for choosing a flow cytometry company
A capable flow cytometry company will have a diverse team of people working multiple functions to support their customers. Ensure the flow cytometry company you do business with offers:
- Multiple, complementary and interdependent functional teams beyond service engineers
- A global Infrastructure with the resources and capability to service each customer as if they were their only customer
- A team of people—the most impactful, sustainable aftermarket care program of a manufacturer is a customer-centric, resource-rich team
High-performance service and support teams
- Field Service Engineers—the front line of installation and repair
- Engineers—creative software and mechanical systems experts devising better ways to do flow cytometry
- R&D—bench scientists developing and validating high-performance antibodies and reagents
- Application Scientists—high-aptitude research scientists with proficiencies across a diverse palette of techniques and applications
- Technical Assistance Group—problem-solving, tribal-knowledge keepers who interact with
- Logistics—masters of getting everything from A to B to you
- Product Managers—orchestrating portfolio development to expand options for customers
- Technical Sales Specialists—partners who identify and customize best-fitting solutions and configurations now and over time
- Market Development & Customer Education—content managers who curate volumes of learning resources
- Executive Leadership—team builders who lead the team to goal achievement and authentic camaraderie
Think beyond the instrument
Ask: How comprehensive is the portfolio?
Why you should ask: Multiple advantages are associated with narrowing the diversity of brands and manufacturers from which you order to manage your lab, including:
- Orchestration—increased instrument, component, and software compatibility for faster set up and implementation
- Operation—convenience and advantageous pricing in ordering and service
- Orientation—increased consistency across staff and operator training, protocol, systems, and maintenance
Critical Consideration: Repair
Breakdown, glitches, and irretrievable clogs can require repair, creating a standstill and costing stalled output, lost samples, wasted sample prep time, and repeated assays. Selecting a flow cytometer with quality engineering, craftsmanship, and hardware (e.g., high-end pumps and lasers), can help reduce the frequency of needed repairs and boost the instrument’s performance and lifespan. Additionally, a competent service and support team can shorten the time to recover and reinstate operation when there is a problem requiring repair.
Characteristics of a strong service-focused team
- Sense of urgency—Responsiveness of service is critical, especially in environments already subject to unpredictable timing and long runtimes.
- Retention of a strong technical competency base—Bench-experienced, credentialed field service engineers, technical assistance partners, complex application specialists, and training officers
- Efficient execution and escalation—Swift delivery of all components, consistency in interactions and protocol
Repair discussion questions
- Along with response time, how long on average are repair tickets open—especially in cases where multiple attempts are made to complete the repair? What is your average speed to resolve?
- Are repair parts new?
- Are repair parts manufactured by the same vendors that supply the parts for the initial unit?
- Are you able to remotely log into our system using internal remote performance sensors and help us troubleshoot?
- Are repairs handled by outside contractors, or are all of your field service engineers employees?
- Do you offer timeframe guarantees for the dispatch of a field service engineer?
- How many different types of instruments are your field service engineers responsible for understanding? Are any dedicated completely to flow cytometers?
- How often are your repair engineers retrained on this specific type of instrument?
- If a field service engineer replaces a part but the instrument still doesn’t work, is our open ticket escalated?
- If a ticket is escalated, then who shows up to fix the instrument?
- If the instrument goes down, will you send a replacement or a loaner? When?
- What extended repair hours do you offer? Will my middle-of-the-night call be routed to middle-of-the-day tech support staff in another time zone?
- What is the territory size, availability, and experience level of the field service engineer who will be assigned to our account?
- Will we have a dedicated repair technician?
Professional maintenance
- How extensive is the scheduled routine professional maintenance?
- How often is maintenance needed?
- Are any routine visits from a field service engineer included in the purchase?
Critical Consideration: Training
With any technology, you have to know how to use it correctly. Flow cytometry can be challenging at first, but the basics are used across all platforms. As new users learn about panel design, compensation, fluorophore selection, and other core skills, they can progressively increase the complexity of their experiments. When selecting an instrument, find out what in-person training, online educational materials, and user communities are available through the manufacturer.
Characteristics of a strong, service-focused team
- Fully-operative customer onboarding program
- Plentiful and generous educational and training resources
- Quality, academically-credentialed trainers
Usability is a relative term, and correlates to the extent of a person’s knowledge of and experience with flow technique, the instrument’s software UX design, the user’s technological aptitude, the relationship between the limitations of their detection equipment and their biology, and the objectives of their experiments. A training program should address these nuances and be able to service beginners, as well as competently laisse with advanced users.
Training discussion questions
- Do you hold user meetings?
- Do you offer active, hands-on training for topics such as panel design and compensation?
- Do you offer additional, comprehensive training? How much does it cost?
- Following the training, will we receive a training manual to keep? If so, how comprehensive is the material?
- Where and how soon after installation will training be scheduled?
- Is the initial training in-person or online?
- If training happens in a facility, will we be learning on the same version of software that comes with the instrument?
- Is ongoing training offered as we get new users?
- What level of complexity is the offered curriculum: beginning, intermediate, or advanced?
- Who is responsible for training new users at a user training center or in their own labs?
- Who trains the trainer at your company?
Decision Workbook: Choosing an extraordinary flow cytometer
This digital workbook features the experimental steps to compare instruments from different manufacturers head to head and a model for how to approach group decision-making dynamics and group accountability in order to reach a consensus supported by measurements plus dialogue.
Resources
Gain familiarity with flow cytometry. These resources will help you increase your understanding of the fundamentals and scientific principles of flow cytometry, and expand your abilities for specialty applications and techniques.











