Search Thermo Fisher Scientific
Sourcing enzymes for mRNA production
How an enzyme supplier can help or hinder your development of vaccines and therapeutics
The success of mRNA vaccines in combating SARS-CoV-2 has paved the way to expand mRNA vaccines and therapeutics in areas like protein replacement therapy, cell and gene therapy, and treatments for infectious and non-infectious diseases. In the development of mRNA vaccines and therapeutics, mRNA production is a critical step and relies on in vitro transcription (IVT) by enzymatic reactions. Selecting enzymes that meet quality standards, regulatory requirements, and documentation support can tremendously accelerate your mRNA drug development from process development to clinical studies and commercialization.

Key enzymes used in mRNA production
First, let’s briefly review the mRNA production process, where enzymes play a critical role. IVT is a cell-free enzymatic process commonly used to synthesize mRNA for vaccines and therapeutics. The steps of mRNA production include template generation, mRNA synthesis, mRNA cleanup, and modification. Each step requires different enzymes for specific purposes (Figure 1 and Table 1).
Figure 1. Enzymes in mRNA production.
Table 1. Key enzymes or proteins used in mRNA production.
| Enzyme or protein | Purpose |
|---|---|
| Template generation | |
| Restriction endonucleases | Linearization of plasmid DNA |
| DNA polymerases | Synthesis of liner DNA by PCR or isothermal amplification |
| RNA synthesis | |
| RNA polymerases | Synthesis of RNA from linear template DNA |
| Pyrophosphatases | Removal of byproducts from RNA synthesis |
| RNase inhibitors | Prevention of RNA degradation |
| RNA cleanup | |
| DNase I | Removal of template DNA |
| RNA modification | |
| Capping enzymes | Capping of synthesized RNA at the 5′ end with an m7G analog |
| 2′-O-methyltransferases | Methylation of capped RNA |
| Poly(A) polymerases | Tailing of synthesized RNA at the 3′ end with adenosines |
Enzyme attributes to consider for mRNA production
There are some important criteria to consider when you are selecting enzymes to produce mRNA—from process development to clinical studies and manufacturing. Ask the supplier questions about the enzymes, such as:
- Will they meet my quality and activity requirements?
- Will they deliver lot-to-lot consistency throughout my process?
- Will they be available in large-scale quantities when I need to scale up?
- Will they be provided with regulatory documentation and support when I need them?
Animal origin–free (AOF) is a typical requirement for enzymes used in the development and manufacturing of mRNA vaccines and therapeutics. AOF manufacturing helps ensure material traceability, identity, and consistency while avoiding risky animal components such as transmissible spongiform encephalopathy (TSE) and bovine spongiform encephalopathy (BSE).
Case study: Breaking new ground on animal origin–free reagents
Read about how Thermo Fisher Scientific was able to quickly develop and execute manufacturing processes to deliver AOF enzymes at scale to a biopharmaceutical client.
To comply with AOF manufacturing, enzymes for therapeutic development are produced from microorganisms using recombinant DNA technology. Nevertheless, contaminants can be carried over from the host cells, as well as introduced from the environment and human operators during the manufacturing process (Figure 2). Host-cell proteins (HCPs) and endotoxins should be minimized, while host-cell enzymes (HCEs), like exonucleases and endonucleases, should be undetectable in final products. For mRNA production, contaminating nucleases and nucleic acids in the enzymes can be detrimental to the process and must be avoided or eliminated as much as possible.
As you scale up mRNA production and move from clinical studies to manufacturing, consistency of enzyme quality becomes more critical to your process. The selected enzymes should be manufactured under a validated process that helps ensure lot-to-lot consistency in quality of the enzymes, regardless of the amount or when you obtain them from the supplier. The quality measures encompass both purity and activity of the enzymes to help you obtain reliable and reproducible products, while minimizing repeated testing as you progress through drug development. To avoid disruption in your development and production, you will need assurance that the supply of the enzymes will be steady and scalable for your demands.
Potential impacts enzyme suppliers can have on your mRNA production
Enzyme suppliers can have a direct impact on quality, consistency, scalability, and regulatory approval of your mRNA production. Before partnering, it may be helpful to ask potential suppliers about the following aspects.
- Innovation: How have their enzymes been developed and innovated over the years? What types of quality by design (QbD) and design of experiments (DoE) have been implemented in process development, manufacturing, and assay development of the enzymes for traceability and quality assurance?
- Manufacturing: How are their enzymes being manufactured (e.g., source, process, facility, QMS, analysis)? The enzymes should not only maintain functional activities but also be free of contaminants, like host and foreign nucleic acids, proteins, and nucleases, to help reduce risks and improve effectiveness of mRNA vaccines and therapeutics.
- Documentation: What type of supporting materials are provided with their enzymes in addition to certificates of analysis and origin, such as product stability data, contaminant analyses, and Drug Master Files (DMFs), for regulatory approval?
- Scalability: Are their enzymes available in large scales in the same quality and consistency as small scales for when your mRNA production progresses from process development to clinical studies and commercialization?
- Flexibility: Can their enzymes be customized for formulation, development, and manufacturing, to fit your needs in drug development?
Benefits of choosing Thermo Fisher as your partner for mRNA enzymes
To help advance the development and manufacturing of vaccines and therapeutics, Thermo Fisher has strived to innovate and expand molecular biology enzymes for over 50 years. Through the use of rational design, in vitro molecular evolution, synthetic biology, and in vitro translation, we have developed and manufactured high-performing and fit-for-purpose enzymes at scale for research and further manufacturing since the 1970s.
Figure 3. Molecular evolution—mutagenesis of existing enzymes. The selection process for Thermo Scientific Maxima reverse transcriptase for increased thermal stability is an example of in vitro molecular evolution.
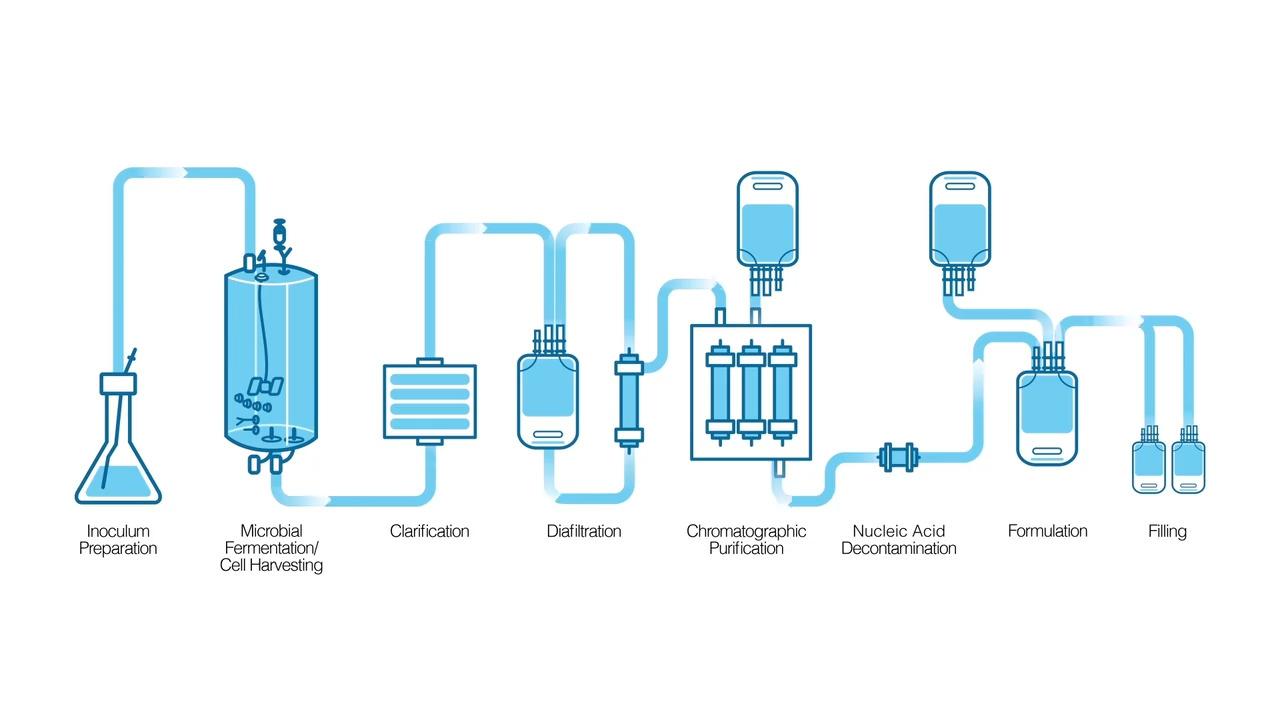
In the mid-2010s, we adapted single-use technology for the novel production of enzymes, similar to that of the biopharmaceutical industry, to drastically minimize the risk of nucleic acid contamination inherent to the conventional manufacturing process. All stages of enzyme manufacturing utilize disposable single-use bioprocessing systems in which single-use components—fermentors, containers and bags, filters, and chromatography columns—are connected by sterile single-use tubes. Buffers and washing solutions are prepared in single-use bags and filtered for sterilization. A 100% closed system helps ensure that the entire manufacturing process is never exposed to the surrounding environment and human operators. Since a single-use system (SUS) does not depend on common-use equipment, the enzyme preparation is protected from potential cross-contamination. Additional proprietary steps were added to remove the majority of host-cell DNA in the early stage of production, and the last traces of host-cell DNA and RNA are trapped using the nucleic acid decontamination step after chromatographic purification.
Furthermore, enzymes manufactured utilizing our SUS technology are subjected to rigorous quality control testing to help ensure their conformity to activity and purity specifications.
- First, functional assays evaluate protein activity to confirm that these enzymes retain the same functional characteristics as enzymes produced by conventional methods.
- Second, enzyme purity is tested to verify that nucleases and contaminating DNA and RNA are not present. Proprietary quality control tests, relying on highly sensitive qPCR assays, are used to confirm that nucleic acid contaminants are absent.
Since the early 2020s, we have been offering AOF enzymes—manufactured using SUS technology under ISO 13485 quality standards, and following relevant ICH Q7 GMP principles—to biopharmaceutical customers for mRNA production, helping them reduce risk and regulatory burden in clinical trials and manufacturing of mRNA vaccines. Moreover, we are actively supporting the development and production of custom enzymes tailored to your specifications for unique purposes and applications. By partnering with Thermo Fisher, you will gain access to our broad portfolio of on-shelf enzymes and custom services—supported by world-class manufacturing capabilities, validated manufacturing processes, comprehensive quality systems, and extensive documentation—to help accelerate the development and manufacturing of your mRNA vaccines and therapeutics.
For Research Use or Further Manufacturing. Not for diagnostic use or direct administration into humans or animals.