Search Thermo Fisher Scientific
TaqMan Mutation Detection Assays

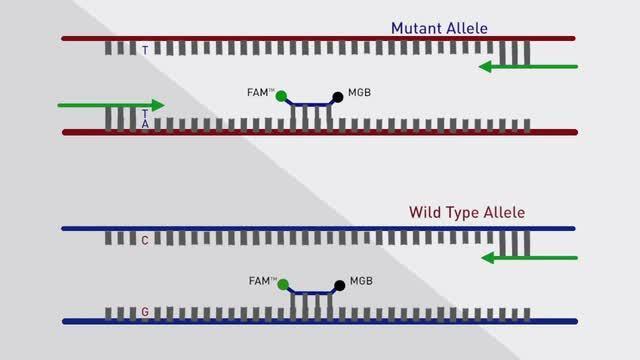
TaqMan™ Mutation Detection Assays are powered by competitive allele-specific TaqMan™ PCR (castPCR™ Technology) to detect and measure somatic mutations in genes associated with cancer research. The castPCR technology is highly specific and sensitive, and can detect rare amounts of mutated DNA in a sample that contains large amounts of normal, wild-type DNA. These assays are compatible with different sample types, such as cell lines, formalin-fixed paraffin-embedded (FFPE) tissue samples, and fresh frozen tissue samples.

castPCR technology
Competitive, allele-specific TaqMan PCR utilizes an allele-specific primer for mutant allele detection that competes with an MGB blocker oligonucleotide to suppress the wild-type background.
By combining the power of TaqMan and castPCR technologies, TaqMan Mutation Detection Assays give you:
- Higher specificity—designed to block the wild-type and amplify only the mutant
- Higher sensitivity—designed to detect somatic mutations down to 1 cancer cell in 1,000 normal cells
- Faster workflow—3 hours from sample to result
With 819 assays for 47 known cancer research genes available today like KRAS, BRAF, KIT, JAK2, the TaqMan Mutation Detection Assays provide an innovative tool for your cancer research.
The TaqMan™ Mutation Detection IPC Reagent Kit is a set of optional internal positive control reagents that can be duplexed with any TaqMan Mutation Detection Assay to provide a positive PCR control result. The TaqMan Mutation Detection IPC Reagent Kit is pre-optimized for use with the TaqMan Mutation Detection Assays.
Order your TaqMan Mutation Detection Assays pre-plated into Fast or standard 96- or 384-well plates with the TaqMan™ Custom Plating Service. Save time and reduce the resources needed for large-scale experiments.
For data analysis, Applied Biosystems™ Mutation Detector™ Software allows users to determine the mutation status of their samples from TaqMan Mutation Detection Assay data collected on Applied Biosystems™ Real-Time PCR Systems.
TaqMan Assay documentation files
The TaqMan documentation files can be downloaded at thermofisher.com/taqmanfiles
Manuals & Protocols
Protocol: TaqMan Mutation Detection Assays
Quick Reference Card: TaqMan Mutation Detection Assays
User Guide: Mutation Detector™ Software
Quick Reference Card: Mutation Detector™ Software
TaqMan Mutation Detection Assay Index File
Product Literature
Product Bulletin: TaqMan Mutation Detection Assays
Application Note: Accurate and sensitive mutation detection and quantitation
Poster: Cancer Biomarker Research Using castPCR Technology
Related applications
Related products
Data Analysis Tools
| Discrimination | castPCR technology combines allele-specific TaqMan qPCR with allele-specific MGB oligonucleotide blocker to effectively suppress non-specific amplification from the off-target allele. |
| Assay Components | Each pre-formulated mutant or wild type allele assay contains: A 10X mix of unlabeled PCR primers, a TaqMan™ MGB probe (FAM™ dye-labeled), and an MGB oligonucleotide blocker Each pre-formulated gene reference assay contains: A 10X mix of unlabeled PCR primers and a TaqMan MGB probe (FAM™ dye-labeled) |
| Reactions | Sufficient for 150 10µl reactions in a 384-well plate or 75 20µl reactions in a 96-well plate. |
| Expiration Date | 2 years from the date of manufacture |
| Assay Information File | Includes sales order number, part number, assay ID, NCBI gene name, and entrez gene ID. Also includes Sanger COSMIC ID, nucleotide mutation, amino acid change, and mutation type. |
| Documentation | Available to download at thermofisher.com/taqmanfiles:
|
| Tracking/Identification |
|
| Shipping and Storage | Shipped at ambient temperature. |
| Catalog # | Product Name | Availability |
|---|---|---|
| 4465804 | TaqMan Mutation Detection Assays (mutant allele assays and wild type allele assays) | Inventoried |
| 4465805 | TaqMan™ EGFR Exon 19 Deletions Assay (mutant allele assay to 19 different EGFR exon 19 deletions) | Inventoried |
| 4465807 | TaqMan Mutation Detection Reference Assays (gene reference assays) | Inventoried |
| 4467538 | TaqMan Mutation Detection IPC Reagent Kit | Inventoried |
TaqMan Assay documentation files
The TaqMan documentation files can be downloaded at thermofisher.com/taqmanfiles
Manuals & Protocols
Protocol: TaqMan Mutation Detection Assays
Quick Reference Card: TaqMan Mutation Detection Assays
User Guide: Mutation Detector™ Software
Quick Reference Card: Mutation Detector™ Software
TaqMan Mutation Detection Assay Index File
Product Literature
Product Bulletin: TaqMan Mutation Detection Assays
Application Note: Accurate and sensitive mutation detection and quantitation
Poster: Cancer Biomarker Research Using castPCR Technology
Related applications
Related products
Data Analysis Tools
| Discrimination | castPCR technology combines allele-specific TaqMan qPCR with allele-specific MGB oligonucleotide blocker to effectively suppress non-specific amplification from the off-target allele. |
| Assay Components | Each pre-formulated mutant or wild type allele assay contains: A 10X mix of unlabeled PCR primers, a TaqMan™ MGB probe (FAM™ dye-labeled), and an MGB oligonucleotide blocker Each pre-formulated gene reference assay contains: A 10X mix of unlabeled PCR primers and a TaqMan MGB probe (FAM™ dye-labeled) |
| Reactions | Sufficient for 150 10µl reactions in a 384-well plate or 75 20µl reactions in a 96-well plate. |
| Expiration Date | 2 years from the date of manufacture |
| Assay Information File | Includes sales order number, part number, assay ID, NCBI gene name, and entrez gene ID. Also includes Sanger COSMIC ID, nucleotide mutation, amino acid change, and mutation type. |
| Documentation | Available to download at thermofisher.com/taqmanfiles:
|
| Tracking/Identification |
|
| Shipping and Storage | Shipped at ambient temperature. |
| Catalog # | Product Name | Availability |
|---|---|---|
| 4465804 | TaqMan Mutation Detection Assays (mutant allele assays and wild type allele assays) | Inventoried |
| 4465805 | TaqMan™ EGFR Exon 19 Deletions Assay (mutant allele assay to 19 different EGFR exon 19 deletions) | Inventoried |
| 4465807 | TaqMan Mutation Detection Reference Assays (gene reference assays) | Inventoried |
| 4467538 | TaqMan Mutation Detection IPC Reagent Kit | Inventoried |
The power of digital PCR can also be used to detect rare mutations for cancer research. Using the QuantStudio™ 3D Digital PCR System and wet-lab validated TaqMan SNP Genotyping Assays, you can target over 100 somatic mutations at a prevalence as low as 0.1%.
For Research Use Only. Not for use in diagnostic procedures.