Search Thermo Fisher Scientific
The Microbiome and its Influence on Human Health
What is the microbiome?
The human body is not just made up of our own cells and genetic material. Microbes, which include bacteria, fungi, archaea, and viruses, inhabit our body and form complex ecosystems in areas such as our skin, mouth, and gut. Trillions of microorganisms are actually contained in the human body, and it is estimated microbes outnumber human cells by 10 to 1 (1). The term microbiome was originally coined by Joshua Lederberg to “signify the ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space and have been all but ignored as determinants of health and disease” (2). Over time, the term microbiome has evolved to not only refer to microbiota, but also to the genetic information and the genomes of the microorganisms themselves.
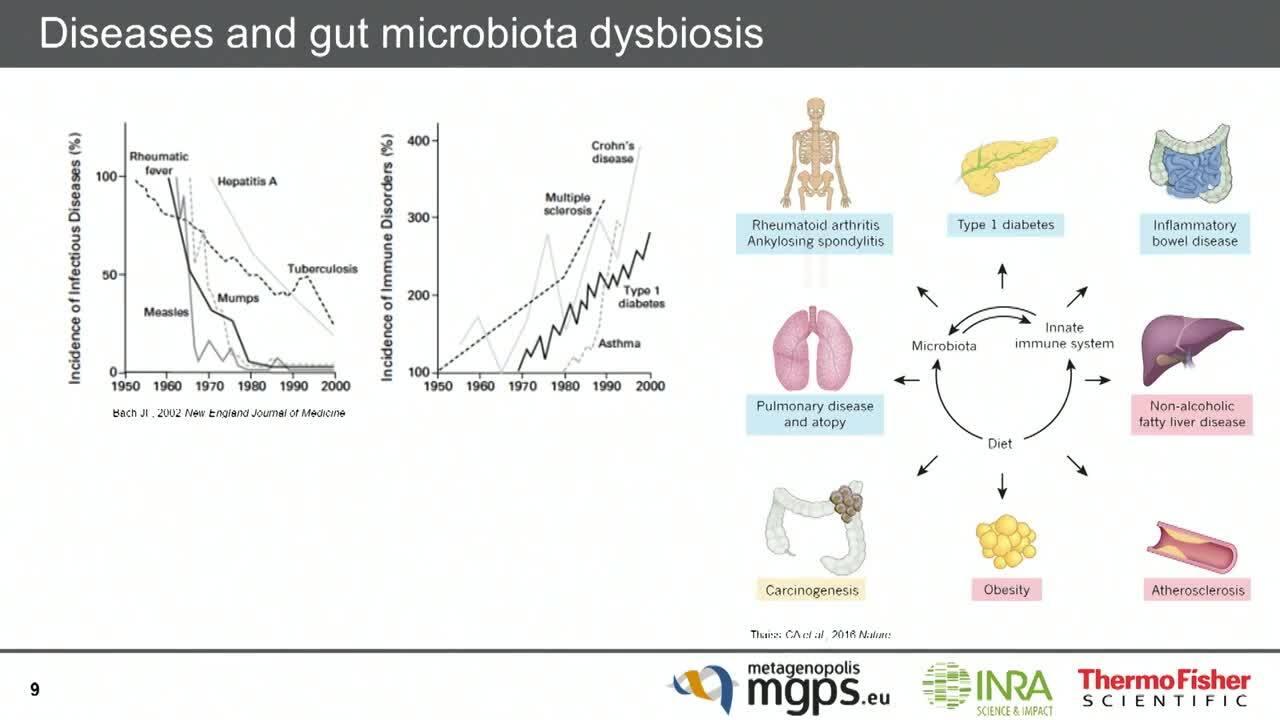
Microbes are no longer studied primarily for their role in causing illnesses. The human microbiome plays a fundamental role in an individual’s well-being. For example, the distal gut microbiome provides us with traits that we did not need to evolve on our own (3). We now know that changes in the microbiome and subsequent changes in interactions with the human body, such as with the immune system, are correlated with a variety of illnesses, including inflammatory bowel diseases (IBDs) and cancer. This microbial imbalance that may lead to disease is called dysbiosis (4).
Featured resources
How does the microbiome impact human health?
It is important to remember that the microbiome is a complex and dynamic ecosystem. The trillions of microorganisms that make up the microbiome vary across environments and over time. A variety of factors, including diet and lifestyle, can influence the human microbiome and thus an individual’s well-being (Table 1).
Factors that influence the microbiome | Example |
| Site on body | Microbial composition varies, even on different parts of the skin with different physical characteristics |
| Diet | Long-term diet has large effects on the gut microbiome |
| Antibiotics | Antibiotics taken early in life affect the gut microbiome and can result in later developmental issues such as obesity |
| Lifestyle | Sleep deprivation correlates with changes in the gut microbiome |
| Genetics | Studies suggest the microbiomes of identical twins are more similar than fraternal twins |
| Pathogens | Can trigger inflammation and alter the composition of the microbiome |
Table 1. Examples of factors that influence the microbiome (5)
When the ecosystem is disturbed and an imbalance results, the microbial colonies that make up the microbiome exhibit a decreased ability to check each other's growth. This can lead to an overgrowth of “bad” colonies, and a vicious cycle may occur where beneficial colonies are further damaged. As the imbalance becomes more pronounced, a pervasive and chronic imbalance between colonies can set in, which affects the mutualistic relationship between the human body and the microbiome. This relationship includes even the metabolic products from the microbiome, as well as interactions with our immune system.
For example, antibodies can disrupt the microbiome within the intestine, resulting in the over growth of Clostridium difficile. Normally, C. difficile is present at low levels and controlled in general by an individual’s healthy microbiome. However, antibiotic disruption can give C. difficile an opportunity to grow without the normal checks and balance. The bacteria produce a toxin that damages the large intestine, causing severe diarrhea and other symptoms (6). Further antibiotic treatment is required to attack C. difficile, however 20% of patients with this infection have a recurrence even after treatment now that their microbiome is imbalanced. Interestingly, this balance has been shown to be restored by performing a fecal microbiota transplant (FMT), where a healthy intestinal microbiome is prepared and transferred to the patient to help address the dysbiosis.
Initial studies of the microbiome were limited due to reliance on classical microbiological methods, such as culture media and microscopy to provide identification. Given many microbes are not culturable, classic techniques would not be able to identify the composition of the multiple microbial communities for an individual. Next-generation sequencing (NGS) has enabled researchers to study the microbiome down to the genomic level and improve our understanding of how microbes impact human health at an unprecedented level.
(Visit Microbiome analysis using next-generation sequencing (NGS) to learn more about the sequencing methods used to study the microbiome.)
The influence of the microbiome on cancer and cancer therapy
With the advent of NGS, we now have the ability to study in detail the link between the gut microbiome and cancer, including its role in immunotherapy. There are numerous hypotheses on how dysbiosis may affect tumorigenesis and tumor growth (Table 2) (7) that may now be understood in more detail by high-throughput sequencing.
Cancer | Examples of proposed mechanisms |
| Liver/biliary tract |
|
| Stomach |
|
| Breast |
|
| Colon |
|
Table 2. Examples of proposed mechanisms for tumorigenesis and/or tumor growth across cancer types due to dysbiosis (adapted from Ref 7)
The gut microbiome actually has an integral relationship to both an individual’s innate and adaptive immune system. The lymphoid tissue associated with the gut represents the largest component of the immune system and influences immune response throughout an individual (8). Thus, the mutualistic relationship with the gut microbiome is critical, to allow for the tolerance of normal flora and to enable the immune system to recognize pathogens that threaten a person’s health.

Given its influence on immunity, the gut microbiome may impact the response and toxicity to various immunotherapies. Multiple studies have now demonstrated that the gut microbiome may modulate response to immune checkpoint blockade (6). Graft-versus-host-disease (GVHD) occurs when donor cells, such as T cells transplanted to fight leukemia, begin to attack the individual’s healthy body cells, resulting in a high mortality rate. Compositional differences in the gut microbiome have been associated with differing rates of development for GVHD (6). Advances in NGS have helped with understanding of the specific components of the microbiome in relation to GVHD. For example, individuals with a high level of Blautia had reduced mortality due to GVHD and this microbe may be beneficial to minimize toxicity from this type of therapy (9).
Summary
The microbiome is a complex ecosystem that is unique to each individual as it changes and adapts due to the various environmental pressures a single person experiences through their lifetime. The microbiome is considered by some an organ in its own right, and we are just beginning to understand how this organ interacts with the body and affects our health.
Researchers are using NGS to understand the genetic composition of the microbiome and providing mechanistic insight on which microbes may be beneficial and which detrimental to one’s health. These NGS studies are now being incorporated into clinical research investigations, and given each microbiome is unique to an individual, this represents how NGS is impacting the future of personalized medicine. As we move forward, one can easily imagine using microbiome analysis with NGS to help define biomarkers and stratify patient populations, which may help improve therapeutic outcomes in the future.
Visit Microbiome analysis using next-generation sequencing (NGS) to learn more about the sequencing methods used to study the microbiome.
References
- NIH Human Microbiome Project defines normal bacterial makeup of the body https://www.nih.gov/news-events/news-releases/nih-human-microbiome-project-defines-normal-bacterial-makeup-body
- Lederberg J, McCray AT. Scientist 15:8 (2001)
- Gill SR, et al. Science 312:1355 (2006)
- Tamboli CP, et al. Gut 53:1 (2004)
- Gilbert JA, et al. Nature Med 24:392 (2018)
- Conrad R. Med Sci Rev 2:92 (2015)
- Helmink BA, et al. Nature Med 25:377 (2019)
- Gopalakrishnan V, et al. Cancer Cell 33:570 (2018)
- Jenq RR, et al. Biol Blood Marrow Transplant 21:1373 (2015)
Additional resources
For Research Use Only. Not for use in diagnostic procedures.