Search Thermo Fisher Scientific
Wohl-Ziegler Bromination

In 1919, German chemist Alfred Wohl investigated the reaction between 2,3-dimethyl-2-butene and N-bromoacetamide in diethyl ether, and discovered that the double bond of the substrate remained unchanged and one of the methyl groups was replaced by bromine. This discovery was interesting, as the reaction was previously thought to require the use of bromine at high temperatures to react with the alkenes. In 1942, Karl Ziegler carried out a comprehensive study of the utility of N-bromosuccinimide (NBS) in the allylic bromination of olefins and demonstrated the synthetic value of the process. Subsequently, the addition of bromine at the allylic position of olefins or at the benzylic position of alkylated aromatic or heteroaromatic compounds became known as the Wohl-Ziegler bromination.
The Wohl-Ziegler bromination has been utilized in several total synthesis campaigns, including James M. Cook’s preparation of (-)-tryprostatin A, a natural product isolated from a marine fungus that inhibits cell cycle progression.
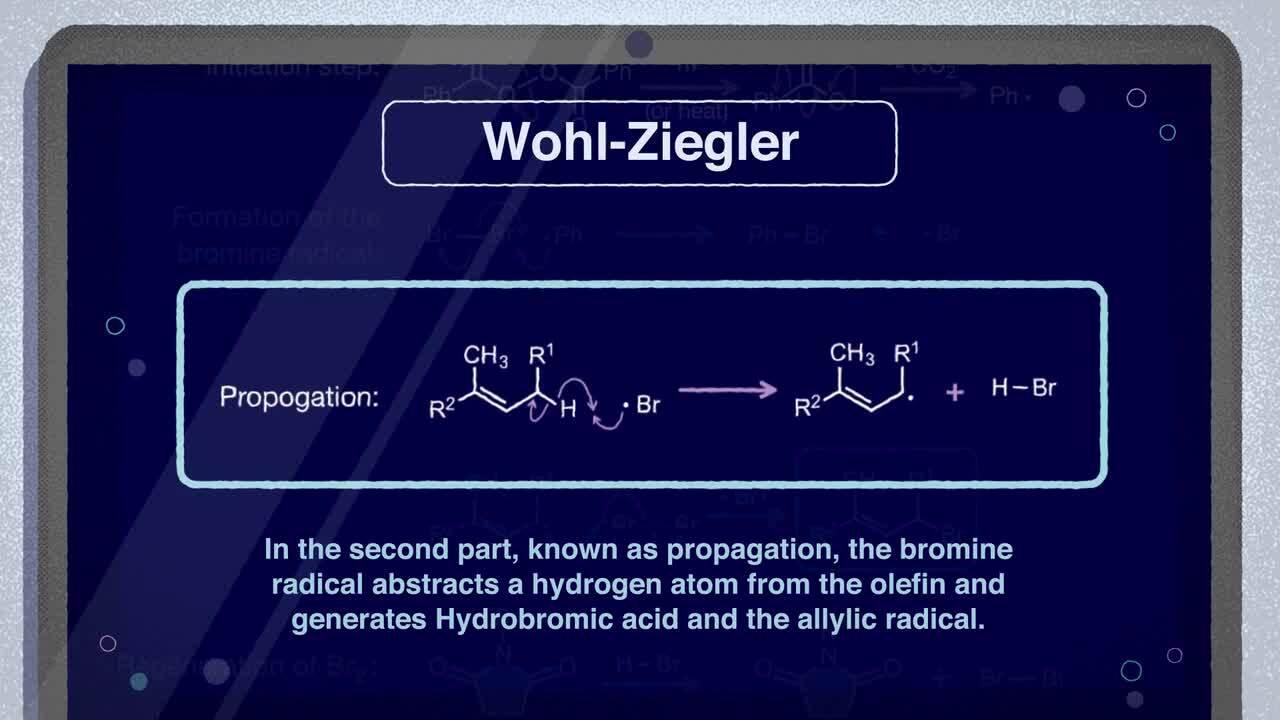
Mechanism of the Wohl-Ziegler bromination reaction
Review available Thermo Scientific products for the Wohl-Ziegler bromination reaction
Resources
Support
For Research Use Only. Not for use in diagnostic or therapeutic procedures.