Search Thermo Fisher Scientific
Discover the power of prenatal screening on KRYPTOR
Are you looking for a complete solution to enhance your prenatal screening capabilities? Look no further. Our comprehensive offering, which includes Thermo Scientific B·R·A·H·M·S Biomarkers, Thermo Scientific B·R·A·H·M·S KRYPTOR Instruments, and Thermo Scientific B·R·A·H·M·S Fast Screen Software, is tailored to meet the needs of clinicians and laboratory managers like you.
Benefit from the power of prenatal screening on KRYPTOR
Explore our complete biomarker portfolio
Our B·R·A·H·M·S Biomarkers offer a wide range of options to cover multiple screening strategies throughout pregnancy. With our solution, you have the flexibility to choose the most suitable biomarkers for your specific screening needs. By utilizing just one instrument, you can streamline your workflow and optimize efficiency in your laboratory.
| Aneuploidy and Neural tube defect screening: | |
| First trimester | Second trimester |
| B·R·A·H·M·S PAPP-A KRYPTOR | B·R·A·H·M·S AFP KRYPTOR |
| B·R·A·H·M·S Free βhCG KRYPTOR | B·R·A·H·M·S Free βhCG KRYPTOR |
| B·R·A·H·M·S PlGF plus KRYPTOR | B·R·A·H·M·S hCG+β KRYPTOR |
| B·R·A·H·M·S AFP KRYPTOR | B·R·A·H·M·S uE3 KRYPTOR |
| B·R·A·H·M·S Inhibin A KRYPTOR | |
| Preeclampsia management |
| Risk stratification |
| B·R·A·H·M·S sFlt-1/PlGF KRYPTOR Test System |
Aneuploidy and neural tube defect screening products do not have FDA clearance for sale in the US.
Trust your results with our B·R·A·H·M·S Prenatal Screening solution
We understand the importance of accurate results in prenatal screening. Our B·R·A·H·M·S KRYPTOR Instruments utilize advanced TRACE technology to deliver reliable and precise measurements, minimizing interference and ensuring the highest level of confidence in your patients' results.
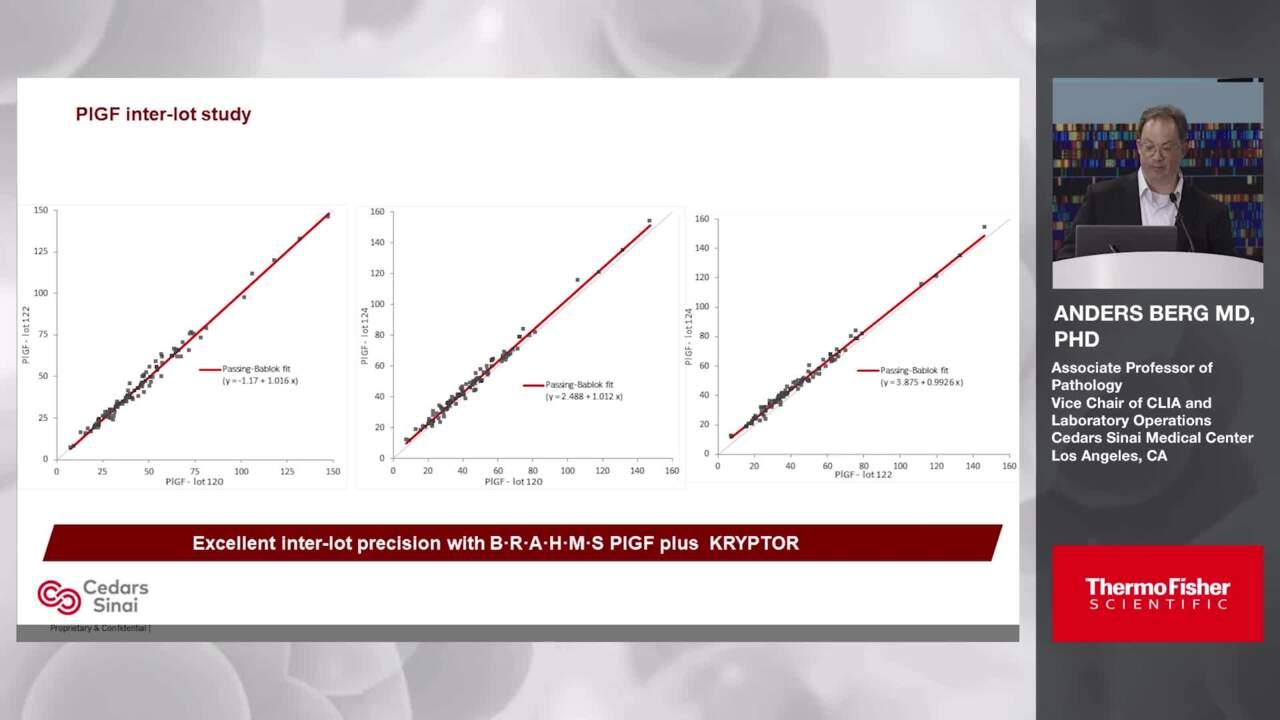
With our biotin-free technology, you can reduce the risk of errors due to interferences and confidently interpret the screening outcomes. Our B·R·A·H·M·S Biomarkers offer outstanding long-term lot-to-lot stability and high precision.3
Not only are our solutions effective, but they are also fully compliant with the new In Vitro Diagnostic Regulation (IVDR) and are approved by the Fetal Medicine Foundation (FMF). Our B·R·A·H·M·S sFlt-1/ PlGF KRYPTOR Test System is the first to receive FDA approval for the risk assessment of preeclampsia with severe features.
This guarantees that our products meet the strictest quality and safety standards, allowing you to focus on delivering the best possible care to your patients.
Get support from the experts
With 25 years of expertise in the field, we have a deep understanding of the complexities involved in prenatal screening and recognize the importance of having access to expert support in your daily practice.
Our dedicated team of experts is providing you with the guidance and assistance you need. From implementation to troubleshooting, we are here to ensure that you have a smooth and successful experience with our solution.
Choose the power of prenatal screening on KRYPTOR and unlock the full potential of your practice. Experience the confidence that comes with our complete solution, backed by years of expertise and unwavering commitment to delivering excellence in prenatal care.
Featured resources
Related pages
References
- World Health Organization. (n.d.). Congenital disorders. World Health Organization. https://www.who.int/health-topics/congenital-anomalies
- Magee LA et al. NEJM 2022;386:1817-32
- UK Neqas reports
Thermo Fisher Scientific products are distributed globally and their uses, applications, indications, claims and availability of products in each country depend on local regulatory marketing authorization status, please consult the Instructions For Use (IFU) available in your country.
© 2024 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified. B·R·A·H·M·S is a registered trademark of B·R·A·H·M·S GmbH.
KRYPTOR and TRACE are trademarks of Cisbio Bioassays, licensed for use by B·R·A·H·M·S GmbH, a part of Thermo Fisher Scientific. Nobel Prize is a registered trademark of the Nobel Foundation.