Search Thermo Fisher Scientific
Blood Cell Counting Using the Countess II FL Automated Cell Counter
There can be numerous challenges when assessing cell health in freshly harvested peripheral blood mononuclear cells (PBMCs, also called white blood cells or WBCs). The hemocytometer is the most commonly used instrument for determining cell concentrations. However, the task is tedious, requires careful cleaning and handling of the hemocytometer, and is subject to variation between users. Furthermore, blood is a rather complex sample type, as it contains various components that can lead to difficulty in manual cell counting. Use of an automated cell counter, such as one of the Invitrogen Countess II Automated Cell Counters, can significantly improve the accuracy of results while saving time over manual cell counting.
Key considerations
When working with PBMC samples, it is important to be aware of some unique properties of these cells. PBMC preparation from whole blood samples requires a technician who is highly skilled in phlebotomy, pipetting, washing, density gradient preparation, and centrifugation set-up. Any error or inconsistency in these steps during the sample preparation process can have a negative impact on the resulting cell sample and count accuracy.
Challenge: Compared to established cell lines, PBMCs can appear relatively faint in brightfield (microscopy) (Figure 1) and be diffi cult to identify using a traditional hemocytometer and microscope, even with trypan blue staining.
Solution: The Countess II instruments automatically detect the optimal focal plane and lighting settings to help improve results when counting PBMCs. It is important to use these automated features to obtain the best results.
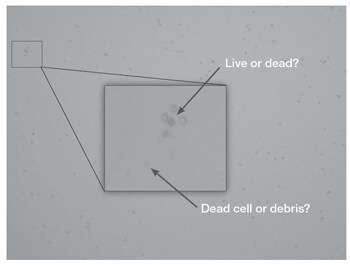
Challenge: Some lysis buffers, including ammonium chloride–based buffers (e.g., ACK lysis buffers), are relatively harsh on cells and can cause false-positive trypan staining immediately after treatment. Red blood cells (RBCs), as well as unhealthy PBMCs, will stain darkly with trypan blue, thus resembling dead cells.
Solution: Our R&D scientists use a Ficoll gradient protocol to ensure removal of RBCs from the sample and preserve PBMC viability.
Challenge: Significant debris may be present due to cell lysis during sample acquisition and processing.
Solution: The debris present can often be omitted from the counts when using the Countess II Automated Cell Counters, by gating out small objects that are less than 5 μm, as shown in Figure 2.
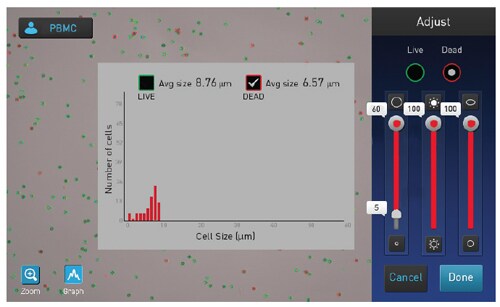
Figure 2. Counting of PBMCs on Countess II instrument. Debris can efficiently be removed from the results by adjusting the cell size slider for the dead cells to gate out items smaller than 5 μm.
Challenge: In unlysed whole blood, there are ~1,000x more RBCs than PBMCs, in addition to a significant amount of debris; hence, your target cells are a very small proportion of the sample.
Solution: Consider removing RBCs via density gradient centrifugation or lysis to enrich for the PBMCs, and then using a nucleic acid stain such as Invitrogen acridine orange, SYTO 9 green fluorescent nucleic acid stain, or ethidium homodimer-1 to stain only nucleated cells. These fluorescent dyes will require an Invitrogen Countess II FL Automated Cell Counter and appropriate filter cubes.
Brightfield vs. fluorescence cell counting
When considering the fluorescent cell counting options currently available, many users ask which counting method is better, brightfield (BF) or fluorescence (FL)? Both have been used for decades and are trusted analysis methods cited in primary literature. The main difference between BF and FL analysis when counting PBMCs is that fluorescence can provide more selective staining, and thus more accurate counts when dealing with complex samples containing significant amounts of platelets, RBCs, or debris. For example, nucleic acid stains such as acridine orange, SYTO 9 stain, and ethidium homodimer-1 will stain only nucleated cells, thereby reducing counting complexity when RBCs and platelets are present. In the same sample analyzed in brightfield and stained with trypan blue, all cellular components present will be observed. Moreover, different fluorescent dyes can be used depending upon the spectral requirements or experimental question being asked; hence, they provide increased flexibility over brightfield counting.
Basic brightfield PBMC counting—general methods
Using a Countess II or Countess II FL Automated Cell Counter and the trusted viability stain trypan blue, you can obtain total cell counts and viability information for your PBMC samples.
Materials
- Either the Countess II (Cat. No. AMQAX1000) or Countess II FL Automated Cell Counter (Cat. No. AMQAF1000)
- Invitrogen Countess Cell Counting Chamber Slides (Cat. No. C10228) or Invitrogen Countess II Reusable Slide (Cat. No. A25750)
- Invitrogen Trypan Blue Stain (Cat. No. T10282)
Protocol: instrument setup
- Turn on the Countess II instrument and select the “Default” profile.
- Install the appropriate slide holder for either the disposable or reusable slide.
Protocol: culture preparation and counting
- Acquire a sample cell suspension and trypan blue.
- Thoroughly mix 10 μL of the cell sample with 10 μL of the trypan blue.
- Apply 10 μL of the stained cell sample mixture to thecounting slide.
- Allow the cells to settle for 30 seconds after loading the sample to help ensure a uniform focal plane and accurate counts.
- Insert the counting slide into the instrument’s sample port to initiate autofocus.
- Press “Count”.
- Press “More” and then “Adjust” for fine control of data selection such as gating out of debris.
- Selecting “Graph” in the lower left of the screen displays the histogram. When using the size gating feature this helps to identify the cell population of interest.
- Debris can be gated by selecting the dead cell radio button and moving down the circularity slider. To omit particles smaller than 5 μm, move up the size slider.
- Multiple gating options can be used together for complex samples.
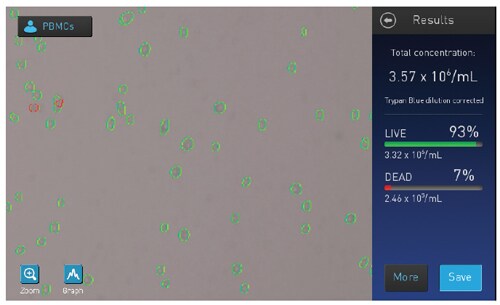
PBMC viability assessment—general methods
Acridine orange (Ex/Em: 500/526 nm) is a cell-permeant nucleic acid binding dye that emits green fluorescence when bound to dsDNA, making it a good stain for total cell counts. The fluorescence emission of the dye when bound to DNA is ~526 nm and can be observed using a standard FITC or GFP filter set.
Ethidium homodimer-1 (Ex/Em: 528/617 nm) is a high-affinity nucleic acid stain that is weakly fluorescent until bound to DNA and emits red fluorescence. Ethidium homodimer-1 is commonly observed using Texas Red filter sets.
Materials
- Countess II FL Automated Cell Counter (Cat. No. AMQAF1000)
- Countess Cell Counting Chamber Slides (Cat. No. C10228) or Reusable Slide (Cat. No. A25750)
- EVOS Light Cube, GFP (Cat. No. AMEP4651) and EVOS Light Cube, Texas Red (Cat. No. AMEP4655)
- Invitrogen Acridine Orange (Cat. No. A3568)
- Invitrogen Ethidium Homodimer-1 (Cat. No. E1169)
Protocol: instrument setup
- Turn on the Countess II FL instrument and install the EVOS GFP and Texas Red Light cube(s).
- Install the appropriate slide holder for either the disposable or reusable slide.
Protocol: culture preparation and counting
- Acquire a cell suspension, acridine orange, and ethidium homodimer-1.
- Stain the cell sample to achieve final concentrations of 5 μg/mL of acridine orange and 3 μg/mL of ethidium homodimer-1.
- Apply 10 μL of the stained cell sample to the counting slide.
- Insert the counting chamber slide into the Countess II FL sample port to initiate autofocus.
- Adjust the FL light intensities to minimize background.
- Press “Count”.
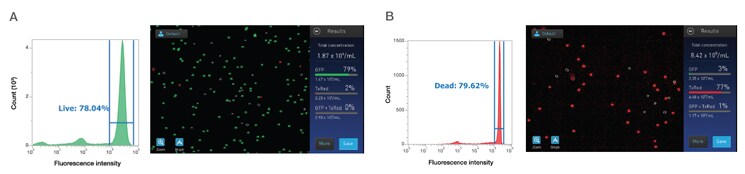
Figure 4. Fluorescent detection of PBMCs compared to flow cytometry. Control (A) or heat-treated (B) PBMCs were stained with 5 μg/mL of acridine orange and 3 μg/mL of ethidium homodimer-1 to label all cells and dead cells, respectively. For each sample, a portion was run in parallel on an Invitrogen Attune NxT Flow Cytometer, where the results from the Countess II FL counter were confirmed.
Summary
Compared to the data obtained by flow cytometry, complementary count and viability data were obtained in the fluorescence cell counting examples shown in Figure 4, despite the potential presence of RBCs, platelets, and debris. Certain conditions must be taken into account when assessing cell health of PBMCs, and the accuracy of results can be improved by using an automated cell counter with appropriate gating strategies and/or fluorescent stains. While acridine orange plus propidium iodide is a commonly used combination, other viability dyes are available, including SYTO 9, Invitrogen 7-AAD, and Invitrogen SYTOX Red Dead Cell Stain.
Resources
Publications
Application notes
Technical resources
Instrument support & demo
For Research Use Only. Not for use in diagnostic procedures.
For Research Use Only. Not for use in diagnostic procedures.