Search Thermo Fisher Scientific
Immune Checkpoint Discovery With Flow Cytometry

Immune checkpoints regulate the immune system’s ability to distinguish self. Tumor cells use certain immune checkpoint pathways as a method of survival, particularly by inhibiting T cell or NK cell activity. Recent therapeutic developments target checkpoints as a method to increase immune surveillance in the tumor microenvironment.
Explore the immune cell–checkpoint interaction with flow cytometry. Quantitate cells that produce biomarkers of inflammation and immune response from a large catalog of antibodies and kits.
How it works: Inactivating immune cells in the tumor microenvironment
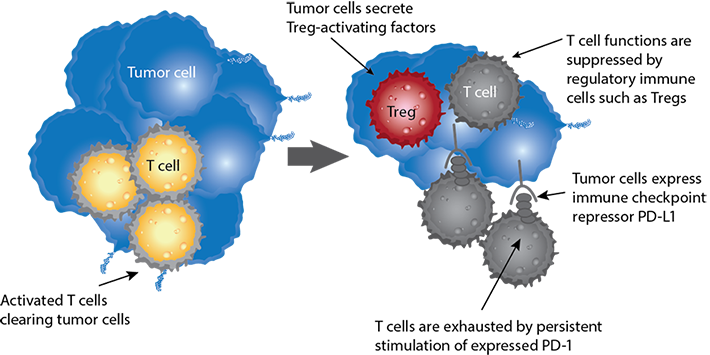
Immune checkpoint flow cytometry workflow
Known targets in immune checkpoint pathways
Antibodies are one tool to probe cellular expression of immune checkpoints. Examine the interaction of cell surface receptors and signal molecules to understand the interaction between cells.
| Immune cell | Effector cell | Function |
|---|---|---|
| Cytotoxic T-lymphocyte– associated antigen 4 (CTLA-4) | T cells | Tumor cells use the CTLA-4 pathway to decrease T cell activation and ability to proliferate into memory T cells |
| Programed cell death protein (PD-1) | T cells | PD-1 expression on T cell indicates exhaustion and inability to perform immune responses |
| OX40 | T cells | Activating OX40 stimulates T cell differentiation and cytolytic function leading to enhanced anti-tumor immunity |
| Glucocorticoid-induced TNFR-related (GITR) | T cells | GITR activation enhances cell reproduction and generate antitumor activity |
| Indoleamine-2,3 dioxygenase (IDO) | Tregs | Tumor cells can upregulate IDO activity through the breakdown tryptophan in order to suppress T cell function |
| CD73 | Tregs | Tumor cells use CD73 to suppress T cell activity with the production of adenosine |
| Lymphocyte-activation gene (LAG) | T cells and Tregs | LAG expression leads to T cell exhaustion and inhibits long-term immune response development |
| CD137 | T cells and NK cells | CD137 stimulates NK cells and T cells for anti-tumor response along with immune memory |
| SLAM family member 7 (SLAMF7) | NK cells | SLAM7 activation stimulates NK cells and other immune cells in the development of long-term immunity |
| Killer-cell immunoglobulin- like receptors (KIR) | NK cells | Tumor cells evade NK cells with KIR expression |
| Programed cell death protein ligand (PD-L1) | Tumor cells | PD-L1 |
Examining inflammation with flow cytometry

Mince and enzymatically digest organ or tissue overnight

Dilute sample

Perform immunophenotyping analyses on the Attune NxT Flow Cytometer
Checkpoint inhibitors may induce off-target inflammation in other organs including the gut or heart. Dilute crude cell suspension from organs and tissues to examine millions of cells quickly with the Attune NxT Flow Cytometer. Look for common inflammation markers including prostaglandins, CXCL13, and IFN-γ.
Measuring T cell activity with flow cytometry
The purpose of checkpoint inhibitors is to prevent T cell exhaustion. Assess immune cell function by detecting cytokine production in tumor samples. T cells and NK cells produce interferon gamma (IFN-γ) to exert an immune response under inflammatory conditions or host defense. Quantitate CD8+ or CD4+ T cells that produce IFN-γ in tumor samples with multi-parametric flow cytometry.

Detecting IFN-γ in a subset of CD4+T-cells and NK cells. Intracellular staining of stimulated mouse splenocytes for IFN-γ (clone XMG1.2) within a minor subset of CD4+ T cells and a larger subset of NK1.1+. Data obtained using the Attune NxT Flow Cytometer.
Resources
Flow Cytometry Learning Center
Access flow cytometry educational resources for better experiment planning and execution.
Product selection
Recommended learning
Support
Flow Cytometry Support Center
Find technical support recommendations for your flow cytometry workflows, including tips for experimental setup and in-depth troubleshooting help.


