Search Thermo Fisher Scientific
Overview of Thermo Scientific 1-Step IVT Systems

The Thermo Scientific 1-Step Human In Vitro Translation System is a method for expressing proteins from DNA or mRNA templates in a cell-free solution containing essential components of the cellular translational machinery. Extracts of an immortalized human cell line provide the ribosomes, initiation and elongation factors, tRNAs and other basic components required for protein synthesis. When supplemented with proprietary accessory proteins, ATP, and an energy regenerating system, these extracts sustain the synthesis of target proteins from DNA templates for up to 6 hours without the need to remove inhibitory by products.
View products
Introduction and protocol summary
1-Step Human In Vitro Translation Kits
The 1-Step Human IVT Kits use human cell lysates to synthesize proteins. Compared to bacterial (E. coli) or other (e.g., insect, wheat germ and rabbit reticulocyte lysates) systems, in vitro protein expression using human cell extracts delivers functional proteins within hours. Expression can be achieved using either a DNA or mRNA templates, which can be derived from a vector or acquired through our library of gene open reading frames.
The benefits of in vitro protein expression include compatibility with microliter-scale reactions and speed compared to traditional cell-based methods (overnight for bacterial cultures or weeks for baculoviral protein preparations). This small-scale expression method makes it easy to express numerous mutant variants simultaneously in a microplate or express large quantities of a single protein for future experiments. Small-scale synthesis also helps to avoid protein aggregation into inclusion bodies, a typical problem for bacterial expression systems. Additionally, expression of proteins in vitro enables synthesis of toxic proteins that can not be produced in live cells. The protein expression system can express protein from any species and has been validated with numerous genes from both prokaryotic and eukaryotic sources.
Highlights:
- Functional – uses the human translational machinery to express active proteins
- Convenient – perform transcription and translation in a single step
- High performance – greater yields compared to rabbit reticulocyte in vitro translation
- Reliable – express proteins that fail in rabbit reticulocyte systems
Better Than Traditional Methods:
- HeLa cell-free extracts are capable of expressing proteins with post-translational modifications
- Accurate translation delivers full-length protein compatible with downstream applications
- Protein translation is optimized with EMCV IRES element and other mRNA stabilizing elements
Selection table of available Human IVT Kits
Available Thermo Scientific 1-Step Human IVT Kits.
| Kit Type | Purpose |
|---|---|
| Coupled IVT | Yields up to 100µg/mL per 25µL reaction in 90 minutes; protocols available for mRNA templates and for optimizing glycoprotein expression. |
| High-Yield IVT | Yields up to 750µg/mL per 0.1mL or 2mL reaction in 6 hours to overnight; uses dialysis devices for continuous feed; protocols available for high throughput (96-well). |
| Heavy Protein IVT | Express proteins with 90 to 95% isotope incorporation in less than 8 hours for use in mass spectrometry as controls for sample prep loss, digestion efficiency determination or as quantification standards. |
View products
Applications and functional studies with human IVT-expressed proteins
Protein function assays for enzymatic activity and protein interactions can have several obstacles. For example, the lack of good antibodies may prevent the identification and verification of proteins in complex samples via co-immunoprecipitation and electromobility supershift assays. Low expression levels can negatively affect the ability to enrich target proteins to sufficient yields for particular assays. These problems can be overcome using in vitro-expressed proteins, but many cell-free protein expression systems lack the ability or fidelity to generate high levels of functional proteins. This problem is compounded for eukaryotic proteins where proper post-translational modifications are critical.
Protein Interactions and the Human IVT System
Understanding the function of a protein often requires an analysis of how it interacts with other biomolecules, such as DNA, RNA and other proteins. Protein-nucleic acid (DNA or RNA) interactions can be monitored by electrophoretic mobility shift assay (EMSA) to help elucidate the regulation of mechanisms from replication to apoptosis. Protein-protein interactions can be analyzed by co-immunoprecipitation and pull-down assays to assist in the identification of novel binding partners.
For protein interaction assays, in vitro-translated protein eliminates the need to extract protein from living cells and facilitates the ability to screen multiple protein isotypes or mutants quickly and efficiently. The following are examples of protein interaction assays performed using recombinant protein generated using the Human IVT System.
A. Protein-Protein Interactions: Co-immunoprecipitation Assays

View products
B. Protein-DNA Interactions Assays: Gel Shift Assays

View products
C. Protein-RNA Interactions Assays: RNA Gel Shift Assays

View products
Protein function and the Human IVT System
In vitro translation reactions are ideal for generating proteins for high-throughput mutational analysis or small-molecule screening. These assays typically require concentrated nanogram to microgram quantities of protein for analysis in multi-well plates. The Human IVT System reactions can be scaled down for 96-well (standard 25µL reaction volume) and 384-well plates (10µL reaction volume) without losing relative protein/mL yield. This feature makes it possible to perform PCR-mediated mutational analysis (see the PCR protocol) and rapidly screen libraries of protein mutants generated by in vitro translation directly in microwell plates.
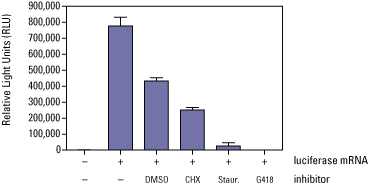
For small-molecule screening, a single protein can be expressed by in vitro translation in multiple wells and then challenged against different small molecules, as demonstrated below using know protein translation inhibitors.
Example: caspase-3 expression and function

View products
Protein types expressed
Traditional in vitro translation systems have limitations in the types of proteins that can be generated, because they based on cell-free extracts from bacteria, insect or other non-mammalian sources that require the addition of ectopic components. The Human IVT System uses human HeLa cell extract, which endogenously contains the enzymes required for protein synthesis and proper post-translational modification. Therefore, many types of proteins that can be expressed using the Human IVT Kits.
View products
Express proteins across a wide range of molecular weights
Successful protein expression is influenced by protein stability, folding and solubility. High molecular weight proteins are difficult to express in vitro because of the greater chance of premature termination and the greater complexity of the tertiary structure compared to smaller proteins, which increases the chance of improper folding [1,2]. Additionally, proteins with greater size can have large hydrophobic regions that promote the formation of inclusion bodies when expressed in E. coli extracts. The 1-Step Human in vitro Translation System can be used to produce a broad range of proteins of different sizes.

Learn more
Express phosphoproteins
The 1-Step Human IVT System uses HeLa cell lysates to perform cell-free protein expression. Using a human-based system yields proteins that mirror natural proteins with respect to post-translational modifications and results in proteins that exhibit natural protein function and structure. Kinase detection by Western blot demonstrates that a protein is expressed by the Human IVT System, but detection of the total protein does not necessarily demonstrate that the protein is functional. Therefore, the phosphorylation status of three kinases (Aurora A, ERK1 and Akt), indicative of functionality, was also confirmed by Western blot using phosphor-specific antibodies. The phosphorylation status of in vitro-translated Akt was also identical in size to that in platelet-derived growth factor (PDGF)-activated NIH3T3 fibroblasts, suggesting that the Human IVT System produces functionally similar kinases to those found in vivo.


View products
Express glycoproteins
The most widely used method of in vitro translation uses rabbit reticulocyte lysate [3]. While this strategy allows 5' cap-independent translation, post-translational modification of nascent proteins such as N-linked glycosylation cannot occur without adding canine microsomal membranes, which are vesicle-like remnants of the endoplasmic reticulum (ER) that form after cells are ruptured (4). Although rabbit reticulocyte lysate with microsomal membranes can generate glycosylated proteins, the approach severely reduces the total protein yield.
An adaptation of the standard protocol for the 1-Step Human Coupled IVT Kit is available, which optimizes for expression of N-linked glycosylated proteins.

Express membrane proteins
Membrane proteins are often the most difficult to purify, because their hydrophobic transmembrane domains can cause large amounts of in vitro-translated protein to aggregate and precipitate. Using the Human in vitro Translation System, though, membrane proteins with varying numbers of transmembrane domains can be synthesized, including the glycophorin GypB/E, the outer mitochondrial membrane protein Bcl2-like protein Bcl2L1 and the chemokine receptor CXCR4 . Additionally, most if not all of the protein was located in the soluble fraction instead of the pellet, demonstrating that the membrane proteins generated do not aggregate and precipitate out of solution.
It is thought that the slower rate of amino acid addition and membrane-enriched HeLa extracts used in the 1-Step Human in vitro Translation System facilitate enhanced protein folding and solubility for membrane proteins. Additionally, the microgram-scale level of protein expression is thought to prevent an increase in the concentration of nascent protein beyond a threshold that drives aggregation that often plagues bacterial expression. Alternative systems using E. coli extracts typically have a much higher rate of amino acid addition, higher protein yields but have lower membrane concentrations, increasing the chance of protein aggregation by reducing folding efficiency [6,7]. Likewise, in vivo expression methods that yield milligram-scale amounts of membrane proteins often result in the formation of inclusion bodies, which are protein aggregates that are time-intensive and difficult to dissociate into soluble protein.

View products
Co-express multiple proteins in the same reaction
Co-expression of different proteins can be performed to assess changes in binding affinity due to mutational analysis or small molecule treatment, and the 1-Step Human IVT System can co-express multiple proteins in a single translation reaction. Indeed, as many as five HA-tagged proteins have been expressed in a single reaction using the Human in vitro Translation System.

For Research Use Only. Not for use in diagnostic procedures.