Search Thermo Fisher Scientific
Purification Using Magnetic Agarose

Magnetic agarose beads consist of highly crosslinked agarose encapsulating a ferrimagnetic core. The beads are 10 to 40 uM in size and have higher binding capacity than traditional magnetic beads. We offer a variety of ligands for immunoprecipitation (IP), co-immunoprecipitation (co-IP), pull-down, and other high throughput affinity screening applications, utilizing immobilized Protein A/G, Ni-NTA, Ni-IMAC (EDTA Compatible), Glutathione, and Anti-FLAG. The beads are removed from the solution manually using a magnetic stand or by automation using an instrument such as the Thermo Scientific KingFisher Flex Magnetic Particle Processor. Automated instruments are especially useful for higher throughput purification and screening of purification conditions.
- High binding—up to 100-fold greater binding capacity than traditional magnetic beads
- More choices—multiple ligands available for different purification strategies
- Automation-compatible—can be used with magnetic particle processors for higher throughput applications
- Flexible—can scale up or down as needed using adaptable protocols
Choose the right purification strategy
| Pierce High-Capacity Protein A MagBeads, alkali stable | Pierce Protein A/G Magnetic Agarose Beads | Pierce Ni-NTA Magnetic Agarose Beads | Pierce High Capacity Ni-IMAC MagBeads, EDTA compatible | Pierce Glutathione Magnetic Agarose Beads | Pierce Anti-DYKD4K (FLAG) Magnetic Agarose Beads | |
|---|---|---|---|---|---|---|
| Binding target | Antibodies | Antibodies | His-tagged proteins | His-tagged proteins, EDTA compatible | GST-tagged proteins | FLAG-tagged proteins |
| Binding capacity | ≥40 mg/mL | ≥40 mg/mL | ≥70 mg/mL | 80 mg/mL | ≥12 mg/mL | ≥3 mg/mL |
| Order products | A53035 A53036 A53037 A53038 | 78609 | 78605 | A50588 | 78601 | A36797 |
Achieve high-throughput sample processing and high protein binding capacity
Poster Presentation: Learn how high-capacity magnetic agarose resin can be automated to achieve high throughput sample processing without sacrificing high protein binding capacity. Dr. Barbara Kaboord, Senior R&D Manager at Thermo Fisher Scientific, demonstrates the successful use of high-capacity magnetic agarose purification workflows for recombinant IgG antibody screening, and for the purification of recombinant proteins expressed in in vitro translation systems.
Easily search and download automated protocols for Pierce magnetic agarose beads using BindIt PC software included with all KingFisher automated purification systems instruments:
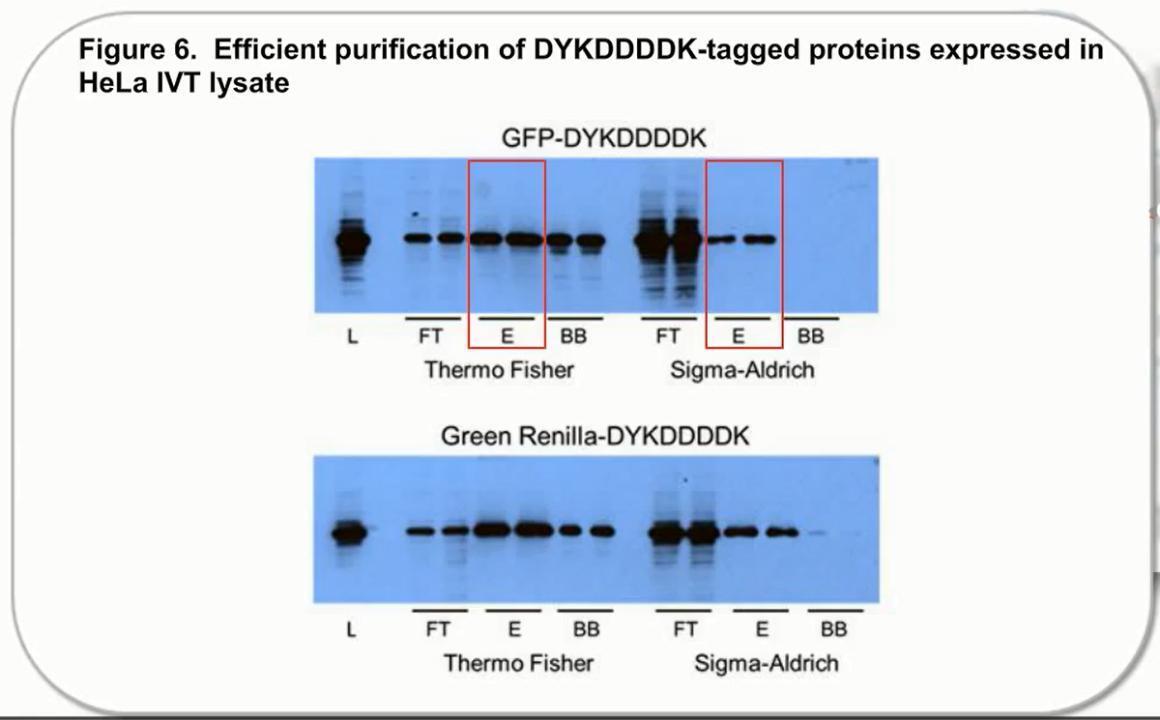
Figure 1. Comparison of protein purification results between Pierce Anti-DYKDDDDK magnetic agarose and another supplier. C-terminal DYKDDDDK-tagged Green Renilla Luciferase protein was expressed using the Thermo Scientific 1-Step Human High-Yield Maxi IVT Kit and immunoprecipitated using Pierce Anti-DYKDDDDK Magnetic Agarose or Sigma-Aldrich Anti-FLAG™ M2 Magnetic Beads using the KingFisher Flex Purification System. Tagged proteins were competitively eluted with Pierce 3x DYKDDDDK peptide and analyzed by western blot (A), and silver staining (C) and Pierce Renilla Luciferase Glow Assay (B). Comparison of the starting lysate (L), elutions (E), and bead boiled samples (BB) show effective capture and elution of DYKDDDDK-tagged proteins with no background. Correlation of protein and activity levels indicate that a high level of Green Renilla luciferase activity is maintained after purification and competitive peptide elution.

Figure 2. Superior performance of Pierce High-Capacity Ni-IMAC MagBeads, EDTA Compatible compared to magnetic beads from supplier C when purifying proteins from cell culture supernatant. Cell culture supernatant (Expi293) containing over-expressed His-tagged EPO (40 mg total protein) was applied to “Pierce High-Capacity Ni-IMAC MagBeads, EDTA Compatible” as well as Competitor C. Beads were processed using protocols with buffers recommended by the manufacturers. Binding was performed with all samples for 30 minutes. The beads were collected on a magnetic stand and the flow-through (unbound) fractions were saved for analysis. The beads were then washed twice, and bound protein was eluted with a 15-minute incubation in Elution Buffer. The eluates were resolved on an SDS-PAGE gel and stained with GelCode Blue (Cat. No. 24594). Gel lanes were normalized by volume. M = MW marker, L = lysate load, FT = flow-through, W = wash, and E = elution.
*HC = High-capacity.
Figure 3. Superior capacity of “Pierce High-Capacity Ni-IMAC MagBeads, EDTA Compatible” compared to magnetic beads from Competitor C, on the Kingfisher Flex. Purified over-expressed 6xHis-GFP was applied to “Pierce High-Capacity Ni-IMAC MagBeads, EDTA Compatible” as well as Competitor C (0-100mg GFP per 10ul settled beads). Beads were processed using protocols with buffers recommended by the manufacturers on the Kingfisher Flex. Binding was performed with all samples for 30 minutes. The beads were collected, and the flow-through (unbound) fractions were saved for analysis. The beads were then washed twice, and bound protein was eluted with a 15-minute incubation in Elution Buffer.
*HC = High-capacity


Figure 4. Comparison of protein yields between Pierce Ni-NTA Magnetic Agarose and products from other suppliers. Samples (0.5 mL) of 6xHis-tagged BirA protein were diluted with 0.5 mL binding buffer and purified manually with 25 mL settled beads. Respective suppliers’ protocols were followed for their buffer compositions and volumes. Pierce Ni-NTA Magnetic Agarose had the highest yield compared to beads from the other suppliers.

Figure 5. Comparison of protein yields between Pierce Glutathione Magnetic Agarose Beads and products from other suppliers. Samples (0.25 mL) of GST-RalGDS were diluted with 0.25 mL binding buffer and purified manually with 25 μL settled beads. Respective suppliers’ protocols were followed for their buffer compositions and volumes. Pierce Glutathione Magnetic Agarose Beads had the highest yield compared to beads from the other suppliers.

Figure 6. Thermo Scientific Pierce Protein A/G Magnetic Agarose Beads provide higher purification yields than other commercially available magnetic beads. IgG purification was performed with the Pierce Protein A/G Magnetic Agarose Beads, GE Healthcare™ Protein A Mag Sepharose Xtra beads, GE Healthcare Protein G Mag Sepharose Xtra beads, Promega™ Magne™ Protein A beads, Promega Magne Protein G beads, GenScript™ Protein A/G MagBeads, BioVision™ Protein A/G Magnetic Beads, and Pierce Protein A/G Magnetic Beads. Mouse and human sera (50 μL) were diluted with binding buffer according to the manufacturers’ protocols and added to magnetic agarose beads. IgG was purified following the manufacturers’ protocols. IgG yield was estimated by absorbance of IgG at 280 nm. All purifications were done in duplicate.
Figure 1. Comparison of protein purification results between Pierce Anti-DYKDDDDK magnetic agarose and another supplier. C-terminal DYKDDDDK-tagged Green Renilla Luciferase protein was expressed using the Thermo Scientific 1-Step Human High-Yield Maxi IVT Kit and immunoprecipitated using Pierce Anti-DYKDDDDK Magnetic Agarose or Sigma-Aldrich Anti-FLAG™ M2 Magnetic Beads using the KingFisher Flex Purification System. Tagged proteins were competitively eluted with Pierce 3x DYKDDDDK peptide and analyzed by western blot (A), and silver staining (C) and Pierce Renilla Luciferase Glow Assay (B). Comparison of the starting lysate (L), elutions (E), and bead boiled samples (BB) show effective capture and elution of DYKDDDDK-tagged proteins with no background. Correlation of protein and activity levels indicate that a high level of Green Renilla luciferase activity is maintained after purification and competitive peptide elution.

Figure 2. Superior performance of Pierce High-Capacity Ni-IMAC MagBeads, EDTA Compatible compared to magnetic beads from supplier C when purifying proteins from cell culture supernatant. Cell culture supernatant (Expi293) containing over-expressed His-tagged EPO (40 mg total protein) was applied to “Pierce High-Capacity Ni-IMAC MagBeads, EDTA Compatible” as well as Competitor C. Beads were processed using protocols with buffers recommended by the manufacturers. Binding was performed with all samples for 30 minutes. The beads were collected on a magnetic stand and the flow-through (unbound) fractions were saved for analysis. The beads were then washed twice, and bound protein was eluted with a 15-minute incubation in Elution Buffer. The eluates were resolved on an SDS-PAGE gel and stained with GelCode Blue (Cat. No. 24594). Gel lanes were normalized by volume. M = MW marker, L = lysate load, FT = flow-through, W = wash, and E = elution.
*HC = High-capacity.
Figure 3. Superior capacity of “Pierce High-Capacity Ni-IMAC MagBeads, EDTA Compatible” compared to magnetic beads from Competitor C, on the Kingfisher Flex. Purified over-expressed 6xHis-GFP was applied to “Pierce High-Capacity Ni-IMAC MagBeads, EDTA Compatible” as well as Competitor C (0-100mg GFP per 10ul settled beads). Beads were processed using protocols with buffers recommended by the manufacturers on the Kingfisher Flex. Binding was performed with all samples for 30 minutes. The beads were collected, and the flow-through (unbound) fractions were saved for analysis. The beads were then washed twice, and bound protein was eluted with a 15-minute incubation in Elution Buffer.
*HC = High-capacity


Figure 4. Comparison of protein yields between Pierce Ni-NTA Magnetic Agarose and products from other suppliers. Samples (0.5 mL) of 6xHis-tagged BirA protein were diluted with 0.5 mL binding buffer and purified manually with 25 mL settled beads. Respective suppliers’ protocols were followed for their buffer compositions and volumes. Pierce Ni-NTA Magnetic Agarose had the highest yield compared to beads from the other suppliers.

Figure 5. Comparison of protein yields between Pierce Glutathione Magnetic Agarose Beads and products from other suppliers. Samples (0.25 mL) of GST-RalGDS were diluted with 0.25 mL binding buffer and purified manually with 25 μL settled beads. Respective suppliers’ protocols were followed for their buffer compositions and volumes. Pierce Glutathione Magnetic Agarose Beads had the highest yield compared to beads from the other suppliers.

Figure 6. Thermo Scientific Pierce Protein A/G Magnetic Agarose Beads provide higher purification yields than other commercially available magnetic beads. IgG purification was performed with the Pierce Protein A/G Magnetic Agarose Beads, GE Healthcare™ Protein A Mag Sepharose Xtra beads, GE Healthcare Protein G Mag Sepharose Xtra beads, Promega™ Magne™ Protein A beads, Promega Magne Protein G beads, GenScript™ Protein A/G MagBeads, BioVision™ Protein A/G Magnetic Beads, and Pierce Protein A/G Magnetic Beads. Mouse and human sera (50 μL) were diluted with binding buffer according to the manufacturers’ protocols and added to magnetic agarose beads. IgG was purified following the manufacturers’ protocols. IgG yield was estimated by absorbance of IgG at 280 nm. All purifications were done in duplicate.
Resources
Learning resources
Poster
Support
For Research Use Only. Not for use in diagnostic procedures.