Search Thermo Fisher Scientific
PSC Resource Handbook – Differentiation
On this page
Request a copy of the PSC Resource Handbook
If you’ve found this chapter – Differentiation – useful, you may be interested in getting your own copy of the entire PSC Resource handbook in either convenient PDF format or print.
The generation of iPSCs is often an intermediate step to reach the real experimental goals. The purpose of PSCs in this case is to take advantage of the proliferative capacity and pluripotency of iPSCs to generate virtually unlimited numbers of mature, differentiated cell types, including neurons, cardiomyocytes, beta (β) cells, or conceivably any other cell type in the body (Figure 5.1). These PSC-derived cells can be used in a range of applications such as:
- Modeling human embryonic development
- As a source of difficult-to-isolate cells for basic research and disease modeling
- Drug screening applications
- Cell replacement therapy

Figure 5.1. Differentiation of PSCs to different lineages via an EB intermediate
More recently, differentiation protocols have become increasingly defined. Most bypass the EB formation step, which effectively created a black box within which signaling events controlling differentiation were poorly understood.
Instead, recent protocols tend towards adherent culture, in which cells are exposed to a temporarily defined combination of small molecules. The replacement of growth factors with potent small molecules allows for differentiation that is not only more cost effective but also more efficient.
As protocols have become more defined, the understanding of signaling events required to specify a given cell type has become increasingly complex. The focus in evaluating differentiation protocols now more typically revolves around the validity and functionality of the generated cells. How accurately does the iPSC-derived cell type recapitulate the behavior of the primary cell in vitro and in vivo? Does it express markers associated with the cell lineage? Does it perform as expected in functional assays? And in many cases, most importantly, does it integrate and function in vivo when transplanted into an animal model?
A final consideration pertains to the maturity of iPSC-derived cells. Cells derived via differentiation of PSCs will by default exhibit a fetal or neonatal phenotype. This may manifest itself via expression of fetus-associated markers, such as fetal globins in iPSC-derived erythrocytes or alpha-fetoprotein in iPSC-derived hepatocytes. Conversely, the expression of adult markers may be low or absent in iPSC-derived cells, such as cytochrome P450 levels in iPSC-derived hepatocytes.
This may be a concern for drug screening and cell therapy applications or for researchers studying late-onset disorders such as neurodegenerative diseases like Alzheimer’s disease or Parkinson’s disease, since disease-specific phenotypes may not manifest themselves in fetal cells.
Ongoing research in this field is exploring ways of aging and maturing iPSC-derived cell types in vitro, and it can be expected that more approaches to this problem will be uncovered in the near future. Until this time, it is recommended that you keep this caveat in mind and plan around its impact on downstream research.
Some of the key considerations to take into account when developing a differentiation protocol, adapting a published protocol from the literature, or choosing a commercially available differentiation kit include:
- High quality of cells—Does the final cell population express the markers associated with the cell type in vivo? Does it perform as expected in functional assays in vitro and in vivo?
- A defined protocol—Does the protocol use defined media and substrates, or does it include components such as serum or BSA? Does it involve an EB formation step or coculture with a stromal cell line? These factors can introduce variability into a differentiation protocol and make standardization and optimization difficult.
- Speed—How quickly is the desired cell population obtained?
- Efficiency—How high is the yield of the desired cell population? Are a considerable number of undesired “contaminating” cell types also obtained?
- Reproducibility—Are cells and efficiencies obtained consistently across multiple experiments and among different users?
- Robustness—Does the protocol work efficiently and consistently across multiple ESC and iPSC lines? Some protocols were developed with a small set of lines and adaptation to different lines may require significant optimization.
- Cost—Does the protocol require significant amounts of expensive recombinant growth factors or specialized tissue culture plates?
- User friendliness—How many different media are required, and how often must cells be passaged or otherwise manipulated? Does the protocol involve labor-intensive picking steps, such as with neural rosettes?
- Scalability—Can the protocol readily be scaled up for the production of high volumes of cells? Is the cost of media prohibitive? Is the culture system with respect to plate format or manual manipulation requirements not amenable to larger scales?
- Bankability—Can cells be frozen as mature cells or at an intermediate stage to establish a bankable population, or must they be derived fresh every time?
- GMP compatibility—If there is interest in potential clinical applications, researchers may want to ask whether a protocol uses GMP reagents or, if not, can readily be converted to one that is GMP-compatible, down the line. Starting with either reagents being manufactured according to GMP or a protocol in which RUO reagents can readily be replaced with GMP reagents may save significant time, effort, and cost. These criteria would be required to adapt and optimize protocols at a later time point when the project is ready to proceed to clinical trials.
View the complete differentiation portfolio at thermofisher.com/differentiation
The derivation of neural cells, including not just neurons but also glial cells such as astrocytes and oligodendrocytes, can be achieved from PSCs via a neural stem cell (NSC) intermediate (Figure 5.2). The NSC is a proliferative population that can readily be expanded and banked for further use. NSCs are multipotent and possess the capacity to give rise to different neuronal subtypes and glial cells, depending on the lineage-specific maturation conditions to which they are exposed. The bankability and multipotency of iPSC-derived NSCs make this an attractive approach for a number of applications including disease modeling, drug discovery, and cell therapy.
Early protocols for NSC specification or induction relied on EB intermediates, stromal co-culture, and/or formation of rosette structures that required manual isolation prior to expansion. These protocols were poorly defined, inefficient, and labor intensive. More recently, protocols relying on adherent cultures differentiated under defined conditions have been developed that allow for rapid and highly efficient induction of NSC populations.

Figure 5.2. The generation of neural cell types from PSCs via an NSC intermediate. Neural specification of PSCs allows for the generation of an expandable NSC population that can further be differentiated to give rise to neurons, astrocytes, and oligodendrocytes via exposure to different lineage-specific signaling factors. Cells at the NSC stage can also be readily cryopreserved, making this a well-suited population for banking large numbers of cells. The media systems to support these different cell populations and transitions are also shown.
Neural induction
PSC Neural Induction Medium is a serum-free medium that provides high-efficiency neural induction of hPSCs in only 7 days (Figure 5.3). Unlike other methodologies, use of PSC Neural Induction Medium does not require the intermediary step of EB formation, thus avoiding added time, labor, and variability.

Figure 5.3. At day 7 of neural induction using Gibco PSC Neural Induction Medium, H9 embryonic stem cell–induced P0 NSCs were dissociated and replated on Geltrex matrix–coated plates overnight. Cells were then fixed and stained with the pluripotent marker Oct4 and neural markers including nestin, Sox2, and Sox1. The replated P0 NSCs were positive for the neural markers nestin (green) and Sox2 (red). Cell nuclei were stained with DAPI (blue).
High-quality NSCs generated using PSC Neural Induction Medium have high expression of NSC markers and can be cryopreserved, expanded, and further differentiated into other neural cell types (Figure 5.4).
For more information, go to thermofisher.com/nscdiff

Figure 5.4. NSCs generated using PSC Neural Induction Medium have high expression of NSC markers and can be cryopreserved, expanded, and further differentiated into other neural cell types.(A, left) Quantification of stained markers showed that less than 1% of P0 NSCs were positive for the pluripotent marker Oct4 and more than 80% of P0 NSCs were positive for the neural markers nestin, Sox1, and Sox2. (B, below) NSCs generated using PSC Neural Induction Medium can be further differentiated into three neural cell types of the central nervous system.

Maturation
To further mature NSCs to specific downstream lineages such as oligodendrocytes, astrocytes, or neuronal subtypes, NSCs must be exposed to additional lineage specific maturation factors. These conditions must be determined and optimized for each cell type. Key signaling pathways involved in lineage specification are summarized in Figure 5.5.

Figure 5.5. Summary of key signaling pathways regulating the differentiation of NSCs to specific neural and neuronal subtypes.
Differentiated neurons from human pluripotent stem cell (hPSC)–derived NSCs enable scientists to study human neural diseases from a large and diverse patient population like never before. However, during and after differentiation, the neuronal cell cultures are typically contaminated with proliferating neural progenitor cells that form clumps and make it nearly impossible to perform critical downstream assays on mature neurons.
Gibco CultureOne Supplement can be added to any conventional neuronal differentiation medium to eliminate more than 75% of contaminating neural progenitor cells with minimal cell death and no effect on kinase-mediated pathways. The resulting superior cultures of evenly distributed, differentiated neurons enable improved downstream assays, accelerated neuronal maturation, and maintenance in culture for 5 weeks or more (Figures 5.6 and 5.7).

Figure 5.6. The addition of CultureOne Supplement allows for superior neural cell cultures. In conventional NSC differentiations, cultures will overgrow and consist of a mixed population of progenitor cells (Sox1) and differentiated neurons (MAP2). Overgrowth of the progenitor cells will lead to clump formation that, with extended maintenance, results in cells peeling off the culture plate. The addition of CultureOne Supplement eliminates progenitors in NSC differentiations, resulting in superior cultures consisting of differentiated neurons. Without overgrowth or clump formation, these cultures can readily be maintained for 5 weeks or longer.
Figure 5.7. CultureOne Supplement enables improved imaging, RNA expression, and electrophysiology assays. After 2 weeks of differentiation with CultureOne Supplement, images show evenly distributed, differentiated neurons (MAP2+) with >75% reduction in NSCs (Sox1+) and cell clumps compared to the conventional differentiation methods (A). These cells had increased neuronal mRNA expression, reduced NSC mRNA expression (B), and exhibited higher spike rates as measured by multi-electrode array (MEA). Neurons differentiated from NSCs with CultureOne Supplement showed an increase in cytosolic calcium when depolarized with KCl (C), meaning they express significantly higher numbers of voltage-gated calcium ion channels, which are an important marker for neuronal maturity and excitability. This, along with the longer neurites after 2 weeks of differentiation, demonstrates that CultureOne Supplement accelerates neuronal maturation.
B-27 Plus Neuronal Culture System
The most cited neural cell culture system consists of Gibco B-27 Supplement and Neurobasal Medium. Originally optimized for long-term culture of rat hippocampal and cortical neurons, this combination has been shown, over two decades of research, to be suitable for a wide range of other neural applications including PSC-derived NSCs and neurons.
However, as the desire for more reliable and biologically relevant models has increased, so too has the necessity for a next-generation media system that can maintain and mature optimal densities of functional neurons over longer periods of time in vitro. The Gibco B-27 Plus Neuronal Culture System features an optimized formulation, upgraded manufacturing process, and more stringent quality control for raw materials and final product. These improvements enable increased neuronal survival by more than 50%, accelerated neurite outgrowth, improved electrophysiological activity, and maturation of neurons (Figures 5.8 and 5.9).
Figure 5.8. B-27 Plus Neuronal Culture System enables superior survival of human stem cell–derived neurons. Cryopreserved HIP Neurons (MTI-GlobalStem) were thawed in classic Gibco Neurobasal Medium with B-27 Supplement and plated onto polyethyleneimine-coated 96-well plates into two volumes of the listed media. Neurons were maintained for 4 weeks with half fluid changes two times per week. Neurons were immunostained with neuronal dendritic marker, MAP2 (green), neuronal cell body marker, HuC/D (red), and nuclei were counterstained with DAPI (blue) (A). Comparability studies indicate that the B-27 Plus Neuronal Culture System is a significantly superior medium compared to the classic B-27–supplemented Neurobasal Medium, with improved neuronal survival and health in long-term cultures (B).
Figure 5.9. Enhanced and accelerated neuronal maturation in B-27 Plus Neuronal Culture System. (A) Rat cortical neurons at day 22 were stained with dendritic marker MAP2 (red), synapsin 1/2 to label presynaptic terminals (green), and DAPI as a counterstain (blue). (B) Neurons maintained in the B-27 Plus Neuronal Culture System had significantly higher numbers of synapsin-positive puncta. (C) Cryopreserved mouse cortical neurons were thawed in classic B-27–supplemented Neurobasal Medium and plated onto poly-D-lysine–coated 96-well plates. Neurons were maintained for ~3 weeks in B-27/ Neurobasal or B-27 Plus/Neurobasal Plus media systems following the suppliers’ recommended protocols. Neurite outgrowth was quantitated on an IncuCyte™ analysis system (Essen BioScience) from differential interference contrast images taken at the time points specified. The B-27 Plus Neuronal Culture System significantly accelerates neurite outgrowth over the first few weeks compared to the classic B-27 and Neurobasal media.
Dopaminergic neuron differentiation
Midbrain dopaminergic (DA) neurons derived from hPSCs provide a viable alternative to primary human neurons for disease modeling and drug screening. While a neuronal population expressing DA markers can be derived from NSCs, studies have shown that it is necessary to proceed via a floor plate intermediate to generate functional, midbrain-specified DA neurons (Kriks et al. (2011); Kirkeby et al. (2012)).
The Gibco PSC Dopaminergic Neuron Differentiation Kit enables the differentiation of pluripotent stem cells (PSCs) to midbrain dopaminergic neurons that secrete dopamine and exhibit spontaneous action potentials. Unlike other protocols or commercially available solutions to differentiate PSCs to dopaminergic neurons (which can be biologically restrictive, lengthy, or ill-defined), our kit enables increased flexibility, speed, and scalability, all while retaining proper biological relevance (Figures 5.10 and 5.11).

Figure 5.10. Simplified workflow diagram. Pluripotent stem cells cultured in Essential 8 Medium can be specified to the midbrain floor plate, expanded and banked, then matured to midbrain dopaminergic neurons in 35 days. Floor plate–derived midbrain progenitors can be expanded up to 10 passages.

Figure 5.11. Representative images of mature DA neurons. The images were obtained from cells stained with reagents provided in the Invitrogen Human Dopaminergic Neuron Immunocytochemistry Kit after 14 days of maturation of floor plate progenitor cells in Dopaminergic Neuron Maturation Medium. The majority of the TH-expressing neurons also coexpressed FoxA2. Cell stains used in these images include anti-TH (green) (A) and anti-FoxA2 (red) and Invitrogen NucBlue stain (a DAPI nuclear DNA stain) (blue) (B). (C) Merged image.
Neuronal functional and cell health assays
iPSCs are powerful tools for disease modeling. They allow researchers to study disease-specific phenotypes in the disease-relevant cell type established from patient-specific iPSCs. The ease with which isogenic controls can be generated via gene editing further allows researchers to eliminate the effects of donor variability and, with high confidence, identify subtle disease-specific phenotypes. However, this requires the availability of assays to interrogate relevant phenotypes.
Neurons are a complex cell type amenable to a variety of cell type–specific assays. Most characteristically, the electrophysiological activity of iPSC-derived neurons can be measured via patch-clamp assays or using multielectrode arrays to determine neuronal subtype–specific AP activity or to assess the effects of neurotoxic compounds.
NSCs can be subjected to a panel of assays compatible with high-throughput methods (Table 5.1) in the presence of various cell stressors to assess neural cell health (Figure 5.12). Additional levels of complexity can be obtained with all of these assays by co-culturing neurons and glial cells to isolate cell-autonomous disease phenotypes from nonautonomous ones, or to determine neuroprotective effects of glia.

Table 5.1. Selected assays that can be used to measure different aspects of neural cell health.

Figure 5.12. A panel of functional assays was used to assess the health of NSCs in response to various cell stressors. iPSC lines were derived from a Parkinson’s disease (PD)-affected donor (PD-3), one multiple systems atrophy (MSA)-affected donor, and two age-matched, and healthy control individuals (Ctrl-1 and Ctrl-2); and differentiated into a NSC population using PSC Neural Induction Medium. The derived NSCs were expanded on Gibco CTS CELLstart Substrate in Neural Expansion Medium for seven passages followed by Gibco StemPro NSC SFM for another four passages. The NSCs were harvested and plated in CTS CELLstart Substrate–coated 384-well assay plates for evaluation by four high-throughput assays. A Tecan Safire reader (Tecan Group Ltd.) was used to measure fluorescence. Representative results are shown for (A) the Invitrogen PrestoBlue assay on Ctrl-2, demonstrating the expected loss in metabolic activity with an increase in the concentration of stressors added, (B) the Invitrogen CellEvent Caspase-3/7 Green assay on MSA, demonstrating the expected increase in apoptosis with an increase in the concentration of stressors added, (C, D) the multiplexed Invitrogen CellROX Green assay and MitoSOX Red assay on PD-3, demonstrating the expected increase in oxidative stress with an increase in the concentration of stressors added.
Few functional behaviors are as impressive as the spontaneous rhythmic contractions of iPSC-derived cardiomyocytes.
Human iPSC-derived cardiomyocytes serve as a particularly important system for studying inherited cardiomyopathies, as studies in animal models have largely been limited by significant differences in human and rodent cardiac electrophysiological properties. It should, however, be noted that iPSC-derived cardiomyocytes exhibit a fetal phenotype.
Applications of iPSC-derived cardiomyocytes include disease modeling, cell replacement therapy (for example following myocardial infarction), and, increasingly, cardiotoxicity screening during drug development.
PSC Cardiomyocyte Differentiation Kit
The Gibco PSC Cardiomyocyte Differentiation Kit consists of a set of serum-free and xeno-free media that enable efficient differentiation of hPSCs to contracting cardiomyocytes in as few as 8 days. Unlike other methods that require multiple components and longer assay duration, the PSC Cardiomyocyte Differentiation Kit can be used to generate cardiomyocytes from PSCs in a ready-to-use media format and in less time.
The kit contains three 1X media that require no thawing or mixing, and each medium is used consecutively over a total of 14 days, resulting in functional cardiomyocytes that express relevant physiological markers, contract in culture, and can be subsequently maintained in culture for more than 15 days.
With optimized conditions, high yields of TNNT2-expressing cardiomyocytes can be generated across a range of ESC and iPSC lines (Figure 5.13). For iPSC lines that may be more difficult to differentiate, our supplemental enrichment protocol based on metabolic selection can improve yield by 10–30%.
You can find both our standard kit protocol and the enrichment protocol at thermofisher.com/cardiacdiff

Figure 5.13. Efficient differentiation across multiple PSC lines. Seeding density is crucial for optimal PSC cardiomyocyte differentiation. PSCs dissociated with Gibco TrypLE reagent were used for setup of these studies. For two lines derived by CytoTune reprogramming, BS2 iPSCs were observed to be promiscuous at higher density. The Gibco Human Episomal iPSC Line (GEPI) was also found to be optimal at a specific density. H9 hESCs were observed to be promiscuous at various densities. The JMP Profiler tool identified optimal seeding densities for efficient differentiation of different PSC lines.
Cardiomyocyte Maintenance Medium
Gibco Cardiomyocyte Maintenance Medium is a serum-free and xeno-free medium that is capable of maintaining cardiomyocytes that have been differentiated using the PSC Cardiomyocyte Differentiation Kit. This medium is included in the kit, but is also sold separately for researchers wanting to maintain differentiated cardiomyocytes in culture for extended periods of time.
Human Cardiomyocyte Immunocytochemistry Kit
The Invitrogen Human Cardiomyocyte Immunocytochemistry Kit enables optimal image-based analysis of two key cardiomyocyte markers: NKX2.5 and TNNT2/cTnT (Figure 5.14). It is the only kit that offers superior imaging reagents for cardiomyocytes in one box, with a complete set of primary and secondary antibodies, a nuclear DNA stain, and all of the premade buffers to enable an optimized staining experiment.

Figure 5.14. iPSCs differentiated for 14 days using the PSC Cardiomyocyte Differentiation Kit. The cells were stained using the Human Cardiomyocyte Immunocytochemistry Kit for the following markers: NKX2.5 (red) for early cardiomesoderm, TNNT2/cTnT (green) for cardiomyocytes, and DAPI for nuclear DNA.
Cardiomyocyte functional assays
The phenotypic and electrophysiological characteristics of iPSC-derived cardiomyocytes are comparable to their primary cell counterparts. The beating syncytium that spontaneously forms is particularly amenable to characterization and analysis. Contractions are accompanied by oscillating intracellular calcium levels that can be measured using calcium-sensitive dyes, and the response to cardiotoxic compounds can be quantified (Figures 5.15 and 5.16). The introduction of disease-associated SNPs via genome editing (see section 4) also enables the use of this system for disease modeling (Figure 5.17).

Figure 5.15. A high-throughput assay for functional measurements on hiPSC-derived cardiomyocytes was developed using cardiomyocytes generated with the PSC Cardiomyocyte Differentiation Kit. After differentiation, cardiomyocytes are replated into 96- or 384-well plates and loaded with Invitrogen fluo-4 dye or FluoVolt probe to measure calcium flux or electrical activity, respectively. Signal transients can then be recorded, analyzed, and turned into meaningful data. This assay can be used for cardiac safety screens or disease model screens.
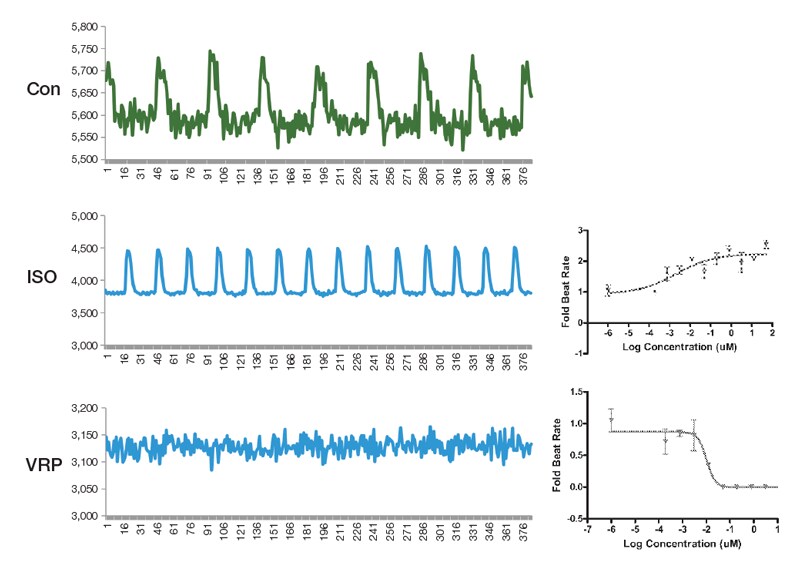
Figure 5.16. Use of the high-throughput functional assay for cardiomyocytes to study the effect of known compounds. Representative fluo-4 traces after treating cardiomyocytes with the β-adrenergic stimulator isoproterenol (ISO) or the calcium channel blocker verapamil (VRP) are shown. Data from these traces can then be used to generate dose-response curves for the different features of a contracting cardiomyocyte such as beat rate as shown.

Definitive endoderm encompasses an intermediate population of cells that gives rise to downstream lineages including pancreas, liver, and gut. As with many other lineages, it has been found that the generation of functionally relevant mature cell types is best achieved through a differentiation protocol that recapitulates the stepwise differentiation during embryonic development, including the passage through a definitive endoderm intermediate.
Downstream lineages have applications in modeling and cell therapy for a wide range of diseases, including diabetes for pancreatic beta cells and metabolic disorders for hepatocytes. iPSC-derived hepatocytes additionally have potential utility for hepatoxicity studies during the drug discovery process.
Traditional protocols for definitive endoderm induction can be costly due to the requirement for activin protein and Wnt signaling.
PSC Definitive Endoderm Induction Kit The Gibco
PSC Definitive Endoderm Induction Kit consists of two xeno-free media that enable efficient induction of hPSCs to definitive endoderm (Figure 5.18). Unlike other methods that require multiple components and take 5 or more days, the PSC Definitive Endoderm Induction Kit enables generation of ≥90% CXCR4+/PDGFRα– definitive endoderm cells with only two components in just 2 days (Figure 5.19).
Each medium is supplied in a 1X complete formulation, requiring no mixing of additional components, and the resultant definitive endoderm shows >90% high expression of the key markers Sox17 and FoxA2 across multiple PSC lines (Figure 5.20) and is capable of differentiating to downstream lineages (Figure 5.21).
Find out more at thermofisher.com/defendo
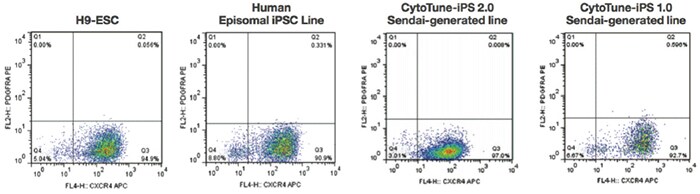
Figure 5.18. The PSC Definitive Endoderm Induction Kit produces definitive endoderm populations with high efficiency (≥90%) across hESC and iPSC lines, including cell lines reprogrammed using episomal vectors or Invitrogen CytoTune kits. Representative dot plots show CXCR4+/ PDGFRα– cell populations derived from various cell lines. For each experiment, unstained cells were used to set quadrant gates.
PSC Definitive Endoderm Induction Kit

STEMdiff Definitive Endoderm Kit

Figure 5.19. Compared to other differentiation protocols, the PSC Definitive Endoderm Induction Kit produces cells in up to 50% less time and requires no predifferentiation or mixing of media.
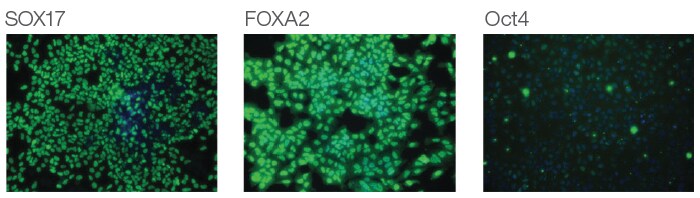
Figure 5.20. Immunocytochemistry of hESCs treated with the PSC Definitive Endoderm Induction Kit. At day 3, induced cells were immunostained for the endodermal transcription factors Sox17 and FoxA2 and the pluripotent marker Oct4. Nuclei were counterstained with DAPI (blue) to assess total cell numbers.
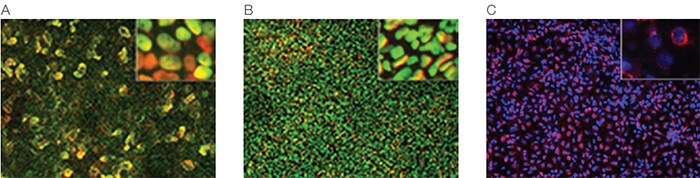
Figure 5.21. Definitive endoderm can be differentiated to downstream lineages. H1 ESCs were treated with the PSC Definitive Endoderm Induction Kit and differentiated into functional cells that express relevant physiological markers: (A) midgut/hindgut (nuclei, blue; FoxA2, green; Cdx2, red); (B) pancreatic endoderm (nuclei, blue; FoxA2, green; Pdx1, red); (C) liver bud progenitors (nuclei, blue; AFP, red). Data credited to LT Ang, KM Loh, and B Lim of the Genome Institute of Singapore.
For Research Use Only. Not for use in diagnostic procedures.



