Search Thermo Fisher Scientific
- Order Status
- Quick Order
-
Don't have an account ? Create Account
Search Thermo Fisher Scientific
CD4+ CD25+ regulatory T cells are a specialized subpopulation of T cells that act to maintain homeostasis within the immune system by suppressing the immune response of other cells. This is an important “self-check” built into the immune system to prevent excessive reactions. Regulatory T cells come in many forms, with the most understood being those that express CD4, CD25, and Foxp3 (CD4+ CD25+ regulatory T cells). Recent advances in the characterization of this cell population have firmly established their critical role in regulating the immune response. Interest in regulatory T cells has been accelerated by evidence from experimental mouse and human models demonstrating that the immunosuppressive potential of these cells can be utilized in research associated with autoimmunity, infectious agents, and cancer [Andersen (2014)].
The Invitrogen Attune NxT Flow Cytometer is available with up to 4 lasers and 16 detection channels. All configurations show excellent separation of cell populations into subsets for immunophenotyping. There is strong signal separation for more data clarity, and up to 14-color detection can be performed with the automated compensation module. This application note describes the use of the Attune NxT Flow Cytometer for 3-color immunophenotyping analysis of stained mouse splenocytes using the Foxp3 Transcription Factor Staining Buffer Kit, a fixation and permeabilization kit designed to work with transcription factors, like Foxp3, and other intracellular markers.
C57BL/6 splenocytes were surface stained with the CD25, CD45.2, and CD4 antibodies listed above, followed by fixation and permeabilization using the Invitrogen Foxp3 Transcription Factor Staining Buffer Kit and intracellular staining with Foxp3 Rat Anti-Mouse mAb, PE Conjugate, or isotype control. The following protocol was used for sample analysis on the Attune NxT Flow Cytometer. Please see the user guide for detailed instructions on setting up an experiment and running samples.
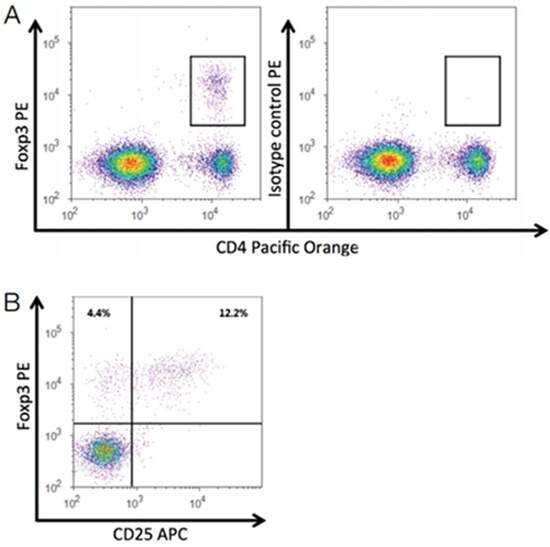
Samples were collected on the Attune NxT Flow Cytometer using 405 nm excitation and the 603/48 nm bandpass emission filter to detect Pacific Orange dye, 561 nm excitation and the 585/16 nm bandpass emission filter to detect PE, and 637 nm excitation and the 670/14 nm bandpass emission filter to detect APC. The gating strategy used in our analysis is described in Figure 1.
Figure 1. Detection of murine regulatory T cells on the Attune NxT Flow Cytometer. (A) Bivariate dot plot depicting the CD4+ Foxp3+ regulatory T cell population (gated) present in mouse spleen (left panel) compared to isotype control (right panel). Cells were gated on lymphocytes based on FSC/SSC profile. (B) CD4+ T cells were gated and analyzed for CD25 and Foxp3 expression. The majority of murine regulatory T cells coexpress the transcription factor Foxp3 and the cell surface marker CD25.
The Attune NxT Flow Cytometer with 4 lasers and 14 colors shows excellent cell population resolution for mouse regulatory T cells consisting of both surface and intracellular markers. This approach can be used to facilitate immunophenotyping studies of regulatory T cells in a variety of sample types.