Search Thermo Fisher Scientific
- Order Status
- Quick Order
-
Don't have an account ? Create Account
Search Thermo Fisher Scientific
For several years, microalgae have been an important source of nutritional products, fuel alternatives, and chemicals. The research into these types of algae for foods, pharmaceuticals, and other chemicals is quickly growing due to the importance of finding more sustainable and less expensive means to produce these products. Depending upon the species, microalgae can be grown in open raceways, photobioreactors, or fermenters. Regardless of the production systems, the maintenance of a healthy culture and the ability to monitor that culture is essential. Flow cytometry is an ideal tool for investigating and monitoring algal cultures. In general, strain improvement in microalgae requires the ability to screen several strains for potential improvement in the target product. The Invitrogen Attune NxT Flow Cytometer offers superior speed and the ability to handle the large and adherent forms of algal culture. In addition, the acoustic focusing technology and Attune NxT Auto Sampler allow for high- throughput screening of strains for improved phenotyping and minimal clogging.
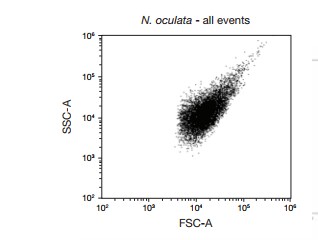
Figure 1. Forward scatter (FSC) vs. side scatter (SSC) dot plot of algae (N. oculata). FSC threshold was set to 5.0 to remove debris from the analysis, FSC voltage was set at 440, and SSC voltage was set at 350.
Figure 2. Determining viability of N. oculata. SYTOX Green stain was added to normal or microwaved cultures of N. oculata, and viability was determined with the Attune NxT Flow Cytometer. (A) Normal culture of N. oculata showing less than 10% SYTOX Green–positive cells (dead cells). (B) N. oculata culture microwaved for 20 seconds, demonstrating that these conditions caused a large increase in dead cells (>95% SYTOX Green–positive cells).
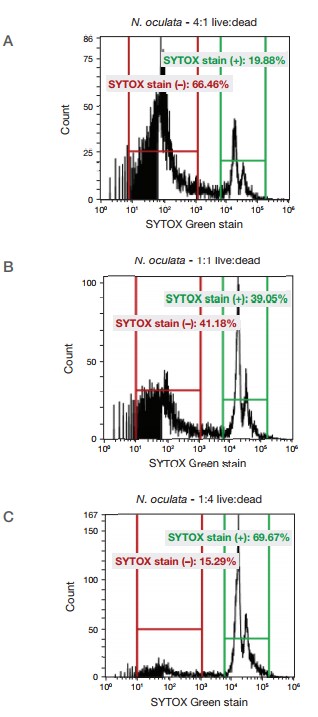
Figure 3. Analysis of mixtures of live and dead cultures of N. oculata. Live and dead (microwaved) cultures of N. oculata were mixed together in different proportions and analyzed with the Attune NxT Flow Cytometer. Viability of cells was determined using the SYTOX Green stain. (A) 4:1 mix of live:dead algal cells. (B) 1:1 mix of live:dead algal cells. (C) 1:4 mix of live:dead algal cells.
Live and dead algae were analyzed with the Attune NxT Flow Cytometer and SYTOX Green Dead Cell Stain. The ability to quantitate different amounts of live and dead algal cells offers a screening tool for laboratories utilizing these types of organisms.