Search Thermo Fisher Scientific
Drug Monographs and Assays Using Ion Chromatography

Modern methods for modern times
In 2011, the U.S. Pharmacopeia (USP) conducted a survey of the international pharmaceutical industry to evaluate existing procedures for the identification of ions and counterions in drug salts. After finding that spectrophotometric tests were the method most commonly being used, the USP undertook an initiative to modernize existing monographs across all compendia. Survey respondents preferred instrumental tests rather than traditional wet-chemistry tests, and expressed an interest in clarification of procedures and acceptance criteria. In addition to meeting these needs, modernizing the monographs will facilitate harmonizing tests with other pharmacopeias, and will enable more rigorous identification tests to decrease adulteration and counterfeiting of drugs.
Advantages of ion chromatography
Existing assays are time consuming, labor-intensive, involve hazardous chemicals, require extensive analyst training, and may yield significant measurement errors. Ion chromatography (IC) offers a significant improvement to these assays, offering the following advantages:
- Speed: IC can simultaneously determine multiple anions or cations in a single injection.
- Reproducibility: IC often uses electrolytically-generated mobile phases (i.e. no manual mobile phase preparation), which simplifies the method and enhances method reproducibility within and between laboratories.
- Accuracy: IC often demonstrates detection limits well below the limits set in the USP monograph.
- Safety: IC can robustly measure multiple analytes without staff handling hazardous reagents.
- Cost-effectiveness: IC uses mobile phases that do not require expensive reagents or pH adjustments.
USP General Chapters
- <1065> Ion Chromatography
- <191> Identification Tests - General
- <345> Assay for Citric Acid/Citrate and Phosphate
- <1225> Validation of Compendial Methods
- <591> Zinc determination
When it comes to IC instrumentation there are a range of technologies available for pharmaceutical applications, including:
- Modular IC systems that allow you to choose from a wide variety of chemistries, detectors, and automation options to fit your advanced application needs.
- Integrated IC systems that feature the reliability you need at the lowest operating cost. Equipped with whole system monitoring and a simplified plumbing layout, even those who are new to IC can quickly become seasoned users.
On either system you'll also save time and improve reproducibility with precise, automatic mobile phase generation and sample preparation.
Speak to a Sales Specialist about Pharmacopeial Modernization
Featured IC learning content
Whiteboard video
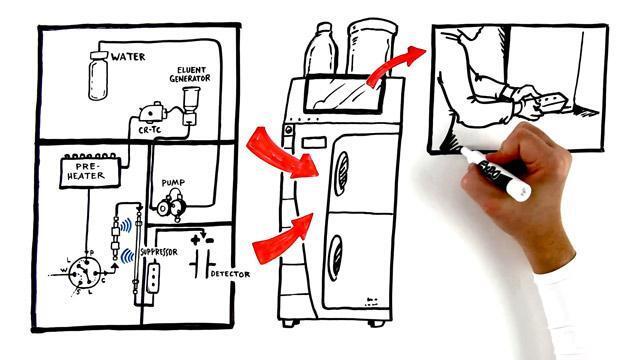
Learn the basics of ion chromatography, including an introduction to the Dionex Integrion HPIC system.
Whiteboard video
This whiteboard video discusses the role of eluents and eluent generation in separating compounds via ion chromatography.
On demand webinar
In this webcast, we will discuss the role of IC in USP monograph modernization, including the presentation of selected case studies where IC has been successfully adopted for monograph modernization.
Modernized IC assays and applications
No records were found matching your criteria