Search Thermo Fisher Scientific
Introduction to cryo-EM
No matter what your stage of research, you can find resources and learning materials you need to overcome obstacles and advance your research with electron microscopy.
Cryo-EM sample preparation guides
Cryo-EM research case studies
Cryo-EM select publications
Fighting SARS-CoV-2 with structural biology methods
In this comment, the authors discuss how advances in the field of structural biology, particularly in single particle cryo-EM and cryo-ET, allow scientists to obtain high-resolution structural information critical for the development of vaccines and therapeutics against emerging pathogens.
Zhang, J., Chen, B. Fighting SARS-CoV-2 with structural biology methods. Nat Methods 19, 381–383 (2022). https://doi.org/10.1038/s41592-022-01448-9
Cryo-EM studies of membrane proteins at 200 kV
In this review, the researchers highlight how 200 kV cryo-TEMs are achieving sub-3 Å resolution structures of membrane proteins and list a number of potential target structures for structure-based drug discovery efforts.
Thangaratnarajah C, Rheinberger J & Paulino C. Cryo-EM studies of membrane proteins at 200 keV. Current Opinion in Structural Biology 76: 102440 (2022). https://doi.org/10.1016/j.sbi.2022.102440
Cryo-EM in molecular and cellular biology
This review summarizes the current state of methods and results achievable by cryo-electron microscopy (cryo-EM).
Saibil HR (2022) Cryo-EM in molecular and cellular biology. Molecular Cell 82 : 274–284 https://doi.org/10.1016/j.molcel.2021.12.016
Cryo-EM methods
Cryo-electron tomography remote data collection and sub-tomogram averaging
This article provides a detailed written and video walk-through of data collection using Tomography 5 and subsequent structure determination using emClarity as it is routinely employed at eBIC (Diamond Lightsource)
AU Sheng Y, AU Morris K, AU Radecke J & AU Zhang P. Cryo-electron Tomography Remote Data Collection and Subtomogram Averaging. JoVE : e63923 (2022). https://doi.org/10.3791/63923
Direct cell extraction of membrane proteins for structure-function analysis
The authors describe a novel method to use cryo-EM for structure-function analyses of membrane proteins extracted directly from cells and incorporated into Salipro lipid nanoparticles.
Drulyte I, Gutgsell AR, Lloris-Garcerá P, Liss M, Geschwindner S, Radjainia M, Frauenfeld J & Löving R (2022) Direct Cell Extraction of Membrane Proteins for Structure-Function Analysis. bioRxiv (2022). https://doi.org/10.1101/2022.07.05.498330
MicroED structure of a protoglobin reactive carbene intermediate
Using the recent technological advances in MicroED, Danelius et al. determined the first structures of Aeropyrum pernix protoglobin (ApePgb) using an AlphaFold2 model for phasing.
Danelius E, Porter NJ, Unge J, Arnold FH & Gonen T. MicroED structure of a protoglobin reactive carbene intermediate. bioRxiv (2022) https://doi.org/10.1101/2022.10.18.512604
Cryo-plasma FIB-SEM volume imaging of biological specimens
The authors describe the use of a cryo-plasma-FIB to produce three dimensional volumes in bacteria, human cells and tissue while retaining the ability to subsequently perform targeted in-situ structural biology using cryo-electron tomography.
Dumoux M, Glen T, Ho EML, Perdigão LMA, Klumpe S, Yee NB -y., Farmer D, Smith JLR, Lai PYA, Bowles W, Kelley R, Plitzko JM, Wu L, Basham M, Clare DK, Siebert CA, Darrow MC, Naismith JH & Grange M. Cryo-plasma FIB/SEM volume imaging of biological specimens. bioRxiv (2022). https://doi.org/10.1101/2022.09.21.508877
Improved AlphaFold modeling with implicit experimental information
The authors describe a method to improve AlphaFold protein structure prediction by iteratively incorporating experimentally determined cryo-EM density maps. This approach could yield a structural model that is more accurate than what can be obtained using either technique on its own.
Terwilliger, T.C., Poon, B.K., Afonine, P.V. et al. Improved AlphaFold modeling with implicit experimental information. Nat Methods 19, 1376–1382 (2022). https://doi.org/10.1038/s41592-022-01645-6
From structure to sequence: antibody discovery using cryo-EM
The authors describe a method to determine monoclonal antibody sequences directly from cryo-electron microscopy-based polyclonal epitope mapping (cryoEMPEM), by combining electron microscopy with next-generation sequencing (NGS).
Aleksandar A, Bowman Charles A, Kirchdoerfer Robert N. et al. From structure to sequence: Antibody discovery using cryoEM. Science Advances 8 : eabk2039 (2022). https://doi.org/10.1126/sciadv.abk2039
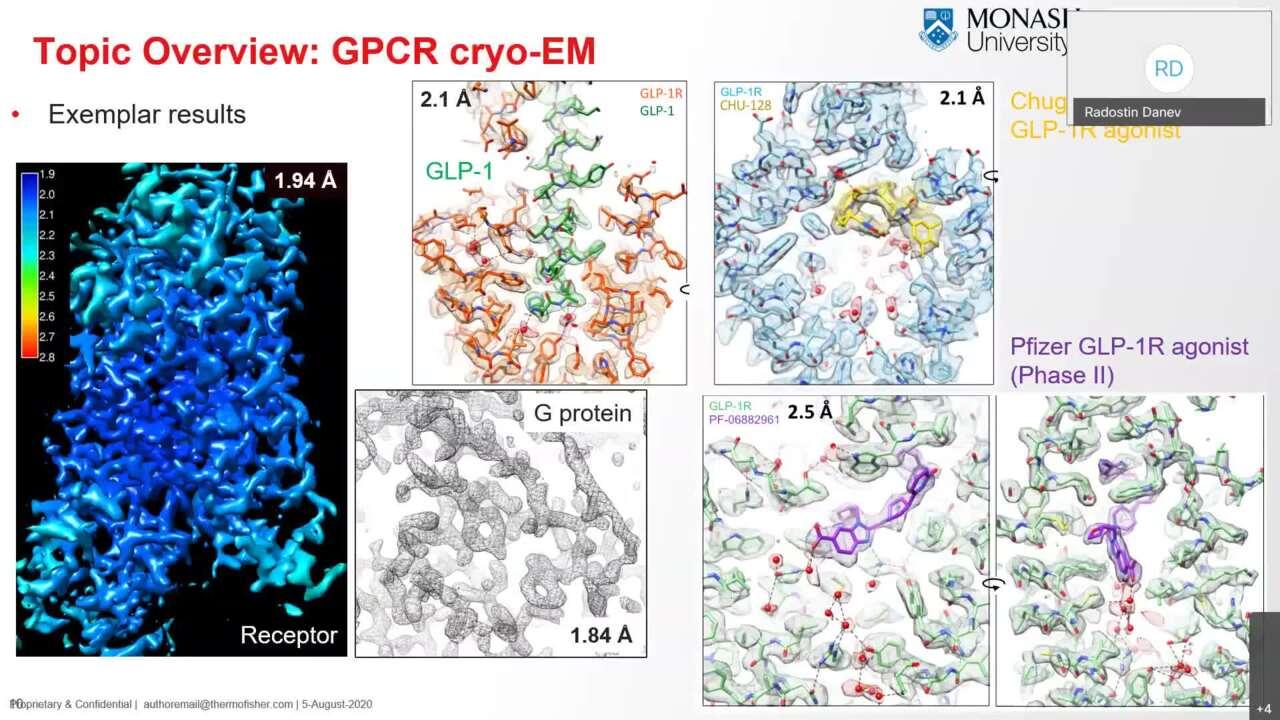
Structure determination of inactive-state GPCRs with a universal nanobody
Here researchers overcome the challenges of obtaining cryo-EM structures of inactive-state GPCRs by using a single-chain camelid antibody (nanobody) yielding resolutions that can exceed those obtained by X-ray crystallography.
Robertson, M.J., Papasergi-Scott, M.M., He, F. et al. Structure determination of inactive-state GPCRs with a universal nanobody. Nat Struct Mol Biol (2022). https://doi.org/10.1038/s41594-022-00859-8 (the preprint can be accessed here: https://doi.org/10.1101/2021.11.02.466983)
Cryo-EM applications
A DNA origami rotary ratchet motor
Using DNA origami, the authors built a rotary ratchet motor at the nanoscale whose mechanical capabilities approach those of molecular motors found in cells. They used single particle cryo-EM to validate the 3D structure of the motor's components.
Pumm, AK., Engelen, W., Kopperger, E. et al. A DNA origami rotary ratchet motor. Nature 607, 492–498 (2022). https://doi.org/10.1038/s41586-022-04910-y
Structural basis for mismatch surveillance by CRISPR-Cas9
In this paper, the authors used single particle cryo-EM to determine conformational intermediates of Cas9 during mismatch cleavage. Their findings support the rational redesign of high fidelity Cas9 variants in the future.
Bravo, J.P.K., Liu, MS., Hibshman, G.N. et al. Structural basis for mismatch surveillance by CRISPR–Cas9. Nature 603, 343–347 (2022). https://doi.org/10.1038/s41586-022-04470-1
Architecture and self-assembly of the jumbo bacteriophage nuclear shell
Laughlin et al., combine cryo-electron tomography and single particle analysis to describe the molecular architecture of jumbo bacteriophage nuclear shell formation.
Laughlin, T.G., Deep, A., Prichard, A.M. et al. Architecture and self-assembly of the jumbo bacteriophage nuclear shell. Nature 608, 429–435 (2022). https://doi.org/10.1038/s41586-022-05013-4
Visualizing translation dynamics at atomic detail inside a bacterial cell
The authors leverage in-situ cryo-ET and advanced image-processing algorithms to determine 13 states of translating ribosomes inside a prokaryotic minimal cell model.
Xue, L., Lenz, S., Zimmermann-Kogadeeva, M. et al. Visualizing translation dynamics at atomic detail inside a bacterial cell. Nature 610, 205–211 (2022). https://doi.org/10.1038/s41586-022-05255-2
A wheat resistosome defines common principles of immune receptor channels
In this publication, the authors report the first cryo-electron microscopy structure of a wheat immune receptor with its fungus-derived effector. Their data indicate the evolutionary conservation of the corresponding receptor family in plants and enables the structure-guided breeding of wheat species that are more resistant against plant diseases.
Förderer, A., Li, E., Lawson, A.W. et al. A wheat resistosome defines common principles of immune receptor channels. Nature 610, 532–539 (2022). https://doi.org/10.1038/s41586-022-05231-w
BacPROTACs mediate targeted protein degradation in bacteria
The authors developed small-molecule degraders in bacteria, so-called BacPROTACs, that could provide a novel way to fight bacterial infections. Single particle cryo-EM was used to visualize the bacterial protease complex in the course of degrading a BacPROTAC-tethered substrate.
Morreale FE, Kleine S, Leodolter J. et al. BacPROTACs mediate targeted protein degradation in bacteria. Cell 185, P2338-2353 (2022). https://doi.org/10.1016/j.cell.2022.05.009
Assembly of recombinant tau into filaments identical to those of Alzheimer’s disease and chronic traumatic encephalopathy
The authors provide in-vitro assembly conditions for recombinant tau filaments that mimic disease-relevant tau folds as determined by ex-situ cryo-EM. A total of 76 in vitro amyloid structures were determined using single particle cryo-EM for this paper, providing a database of structural model systems for future tauopathy research.
Lövestam S, Koh FA, van Knippenberg B, Kotecha A. et al. Assembly of recombinant tau into filaments identical to those of Alzheimer’s disease and chronic traumatic encephalopathy. eLife 11 : e76494 (2022). https://doi.org/10.7554/eLife.76494
The trispecific DARPin ensovibep inhibits diverse SARS-CoV-2 variants
Using single particle cryo-EM, the authors demonstrate how a clinical candidate DARPin antiviral binds the spike protein of diverse SARS-CoV-2 variants to inhibit infection.
Rothenberger, S., Hurdiss, D.L., Walser, M. et al. The trispecific DARPin ensovibep inhibits diverse SARS-CoV-2 variants. Nat Biotechnol (2022). https://doi.org/10.1038/s41587-022-01382-3
For Research Use Only. Not for use in diagnostic procedures.