Search Thermo Fisher Scientific
Charge Variant Analysis Information

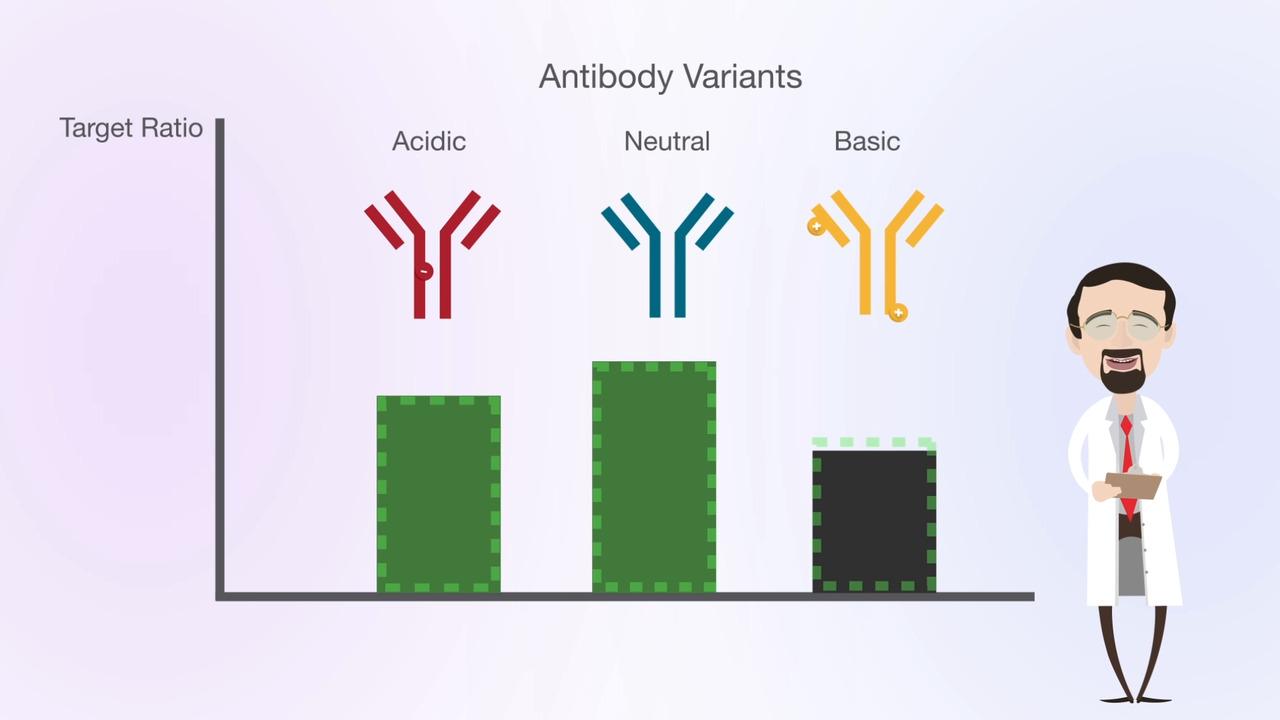
Get structural insight into protein charge variants
The analysis of charged variants is a regulatory requirement for bio-therapeutic proteins. These large heterogeneous molecules can be subject to a variety of enzymatic post-translational modifications during manufacture, such as glycosylation and lysine truncation. In addition, chemical modifications can occur during purification and storage such as oxidation or deamination. Ion exchange charged variant analysis is a high-resolution technique that has proved very useful in the analysis of such variants. Protein charge variant profiling is mandated in regulations such as ICH Q6B .
Protein charge variant analysis by ion exchange chromatography
Protein charge variants can be separated using physicochemical fractionation—based on charge characteristics of antibodies or other proteins. Historically, protein separation has been performed using ion exchange chromatography with salt gradients. A key challenge of salt-based gradients is that a unique gradient needs to be developed for each individual target protein molecule. Moreover, poor reproducibility of salt-based gradients meant that method replication and robustness was often poor and method development was time consuming.
In 2009 Genentech first published the use of pH gradients for charge variant separations instead of salt gradients. The advantages of pH-based gradients include a global applicability of the method to any monoclonal antibodies (mAb), greatly simplifying the method development. It was found that mAbs with isoelectric points in the range of around 7–9 could be well separated using cation exchange columns operated in a pH-based elution mode. Moreover, the approach was more generic and could easily be used to separate the different charge variants in a range of antibodies using a single method, that could also be significantly shorter using pH gradients (30 min.) rather than conventional ionic strength salt gradients (90 min.).
New columns and instrument technologies have shown the potential to decrease the run times even further:
shorter small particle size columns combined with linear pH gradients can bring this analysis into the domain of a true UHPLC application.
A difficulty with this technique is how to produce a truly linear pH gradient. Several buffers have to be employed to cover the whole of the pH range at concentrations so that the buffering capacities of each buffer match each other. The column itself will act as a buffer against any pH changes, so careful selection of a high resolution/low capacity column is required. The difficulty in producing a linear pH gradient with buffer cocktails can be seen clearly from the image below, where a clear curved gradient is produced in response to a programmed linear gradient. This can be overcome through the use of commercially available gradient buffers which, in combination with the correct column, produces a linear gradient. This enables method optimization to be achieved easily and logically and delivers robustness and consistency between operators and laboratories.
Are there alternative techniques?
Isoelectric focusing using capillary electrophoresis (CE) has also been used as a fast global method for charged variant analysis. Whilst this method has shown promise, it is prone to long equilibration times and operator inconsistency. HPLC or UHPLC pH gradients are now faster and more reproducible than CE alternatives. HPLC or UHPLC also have global applicability and familiarity in all biopharmaceutical laboratories. Moreover, HPLC or UHPLC allows the fractionation of any new variant peak detected, so that it can be positively identified with further analysis.
For more information on the products and technologies for protein charge variant analysis please visit our Charge Variant products page.
Featured charge variant profiling learning content
Webinar series
Join scientific experts from the National Institute of Bioprocess Research and Training ﴾NIBRT﴿ in Dublin, Ireland and Thermo Fisher Scientific to hear about the latest analytical advances for intact protein characterization.
Application
In this study salt and pH gradients were evaluated for separation of charge variants of denosumab. The pH buffer gave enhanced separation of charge variants and offered faster screening possibilities.
Application
This study shows that pH gradient–based strong cation exchange chromatography on the ProPac SCX-10 column can provide excellent resolution for MAb charge variants. The variants were partially identified as sialylation variants. Compared to the routine IEF method, this method is more convenient and straightforward for protein quality control.
Protein charge variant profiling literature library
No records were found matching your criteria