Search Thermo Fisher Scientific


Types of HPLC detection methods
After elution from the column, the mobile phase transports separated bands or analytes to the detector – the final HPLC component. While there are many HPLC detection methods, no single one can detect all possible analytes. Liquid chromatographers can utilize two or more detection methods in the same run to obtain deeper sample characterization.
HPLC system components
Related resources
Detection method | Analyte requirements | Detection limit | Destructive detector? |
|---|---|---|---|
UV-Vis (UVD) | Absorbs UV-Vis light between 190 – 800 nm | Nanograms | No |
Fluorescence (FLD) | Has a fluorophore or labelled with a fluorescent tag | Femtograms | No |
Refractive Index (RID) | No analyte restrictions | Micrograms | No |
Evaporative Light Scattering (ELSD) | Non- and semi-volatile analytes | Nanograms | Yes |
Electrochemical (ECD) | Undergoes a redox reaction in the presence of an electric potential | Femtograms | Yes |
Charged Aerosol (CAD) | Non- and semi-volatile analytes | Picograms | Yes |
Mass Spectrometry (MS) | Volatile and semi-volatile ionizable analytes | Picograms | Yes |
You can learn more about the benefits of using multiple detectors in an HPLC analysis
How HPLC detectors work
Most HPLC detectors work by converting a physiochemical property of an analyte into an electrical signal. In other words, a detector ‘sees’ a sample and sends signals at consecutive time points throughout the sample run.
Signal intensity should correlate with the amount – either mass or concentration – of the detected sample at the given time point, allowing the quantification and identification of the separated analytes in a time-dependent manner.
Selecting a detector compatible with your target analytes and separation conditions is crucial when developing a method. If you use a detection method incompatible with the target analytes, you will miss the sample information.
Conversely, some mobile phase compositions or additives can produce noisy backgrounds for specific detectors, preventing proper analyte quantitation.
Types of UV-Vis detectors
For UV-Vis detection, chromatographers can choose between three types of detectors: a variable wavelength detector (VWD), diode array detector (DAD), or multiple wavelength detector (MWD). In variable wavelength detection, a single chosen wavelength from the UV-Vis spectrum illuminates the sample. In contrast, diode array and multiple wavelength detectors exposes the sample to the entire spectrum instead of a single chosen wavelength. The application needs or optical properties of the analyte(s) and sample matrix often determine the detector choice.
How variable wavelength detectors work
A variable wavelength detector uses a rotating grating to disperse polychromatic light into the spectrum. The light of a single wavelength is then selected and passed through the exit slit.
After the light passes through the exit slit, a beam splitter or semipermeable mirror divides the beam into two parts: one part of the light goes to a reference diode to measure the intensity without absorption. The second part passes through the flow cell, where the sample partially absorbs the light. The intensity of the remaining light is measured by the detection photodiode and translated into a quantitative signal.
By selecting a wavelength before exposing the sample, light from one wavelength is used to measure the absorption. This detection method offers high sensitivity due to the simultaneous measurement of an actual reference and reduces the total light exposure of the sample during detection.
The enhanced sensitivity of a VWD versus a DAD is essential for light-sensitive compounds.
How diode array and multiple wavelength detectors work
Diode array and multiple wavelength detectors both use a grating to disperse the light onto a photodiode array after the light has passed through the flow cell. As a result, the absorption of all wavelengths is simultaneous, giving the analyte a full absorption spectrum.
This detection method is preferred when analyzing complex mixtures or samples of unknown composition, for example, during method development or peak purity analysis.
Fluorescence detectors are the most sensitive optical detectors and an excellent alternative to standard, absorption-based UV-Vis detectors for analytes with fluorescent properties or analytes tagged with fluorophores.
The sensitivity can be 10-1000 times higher for fluorescence versus UV-Vis absorption detectors but is limited to fluorescent molecules. For this reason, FLD is less common than UV-Vis.
Fluorescence detectors are exceptionally selective for fluorogenic compounds, and excitation and emission are tunable for a particular class of fluorophore.
How FLD works
Fluorescence detectors work measuring photons emitted by fluorescent molecules after excitation at a particular wavelength.
There is vibrational relaxation before the emission of a photon during the electronic relaxation. This vibrational relaxation leads to the redshift of emitted photons versus the excitation photons, called a Stokes shift.
In simplified terms, fluorescing molecules lose the remaining energy by emitting light higher than the original absorption wavelength.
Refractive index detectors typically measure the deflection of a light beam due to the difference between the refractive indices of the pure mobile phase and the mobile phase containing the analyte. Refractive index detectors are universal detectors, requiring only that the analyte be soluble in the mobile phase.
The downside of RID is sensitivity towards temperature and flow rate as well as eluent composition, which prevents its use with gradient separations. A well-defined thermostatting of the detector and precise flow control is required to maintain sensitivity.
The detection limit for a refractive index detector is considerably lower than UV-Vis and FLD, but there are applications where RIDs should be your first choice.
How RID works
A refractive index is a dimensionless number describing how fast light propagates through a medium compared to the vacuum. Snell's law defines the refraction of light as it crosses the border between media with different refractive indices.
The most common refractive index detector is the deflection type. In this type of detector, the flow cell has a sample cell flow path and a reference cell flow path to compare against the mobile phase.
When an analyte passes through the sample flow cell, the refractive index, and direction of the light inside the flow cell change in proportion to the concentration of the analyte. A subsequent shift in the direction of light intensity is applied to determine the concentration when the system is calibrated for a particular analyte/mobile phase combination.
Electrochemical detectors are selective and extremely sensitive and often used to measure low-level, redox-active analytes in complex biological matrices. Although ECD performance often compares to the sensitivity in FLD, an advantage of electrochemical detection is a direct measurement of an analyte without using complex, time-consuming derivatization procedures.
How ECD works
An electrochemical detector measures the current produced when an electrochemically active compound undergoes oxidation or reduction at the surface of the electrode caused by an applied potential. According to Faraday's law, the resulting current is directly proportional to the concentration of the analyte experiencing the electrochemical reaction.
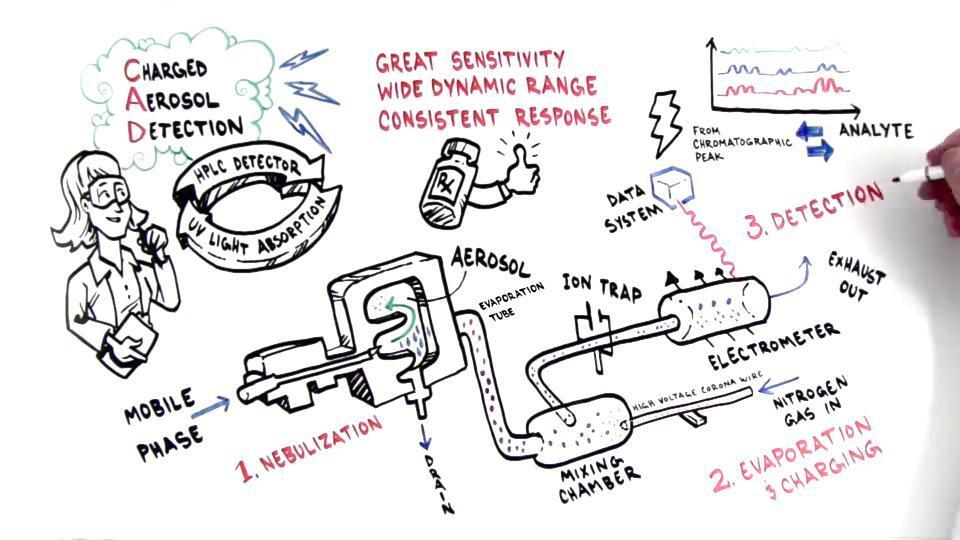
Charged aerosol detectors are near-universal. The high sensitivity, wide dynamic range, and uniform response make CAD excellent for application versatility. The three significant benefits of using CAD are:
Sensitive detection
Independent of whether a chromophore is present or the compound forms gas-phase ionsUniform response
As in the ability to obtain the same response for all components regardless of the analyte structureStandard-free quantitation for unknowns
This functionality is essential when reference standards are unavailable for impurities and degradants
How CAD works
In CAD, analytes detect as a positively charged aerosol, and the total charge measures as a current, independent of optical properties. The size of the charge depends on the particle size, so a greater mass yields a bigger particle with more charge. This large particle size results in higher signal response.
All charged aerosol detectors utilize evaporative technology, and the conversion of an analyte to a detectable signal involves the same successive steps:
Nebulization via droplet formation from an eluent stream
Conditioning by selecting droplets of a certain size range
Evaporation with the conversion of droplets to form residual non-charged aerosol particles composed of non-volatile analytes
Transfer of positive charge from ionized gas to the evaporated aerosol particles
Quantification of the charged aerosol particles by an electrometer
Evaporative light scattering detection (ELSD)
Like charged aerosol detectors, evaporative light scattering detectors are non-linear and near-universal. These detectors measure many semi- and non-volatile analytes and do not require an analyte to possess a chromophore or ionizable group.
How ESLD works
In the ELSD, aerosol detection depends on the light-scattering properties of the analyte, and light intensity is related to the quantity of analyte present. All evaporative light scattering detectors work the same way:
Nebulization via droplet formation from an eluent stream
Evaporation of the eluent leaving behind dried analyte particles
Exposure of dried analyte particles to a beam of light
Scattering of light by dried particles
Measurement of the scattered light by a photomultiplier
Quantification of analyte by photo multiplier tube
Liquid chromatography systems often pair with mass spectrometers. In combination with the retention time from the LC separation, MS detection provides an additional level of information by determining the mass-to-charge ratio of analytes contained in the sample.
Mass spectra contain information regarding the elemental and isotopic composition of analytes, which yields high detection specificity and is helpful for structural elucidation. These detectors are compatible with many analytes capable of forming gas-phase ions, from small inorganic salts to large macromolecules like proteins.
MS detection is more sensitive than other detection methods such as UV-Vis, does not require a chromophore or redox group, and enables the identification and structure elucidation of various molecules.
How mass spectrometry works
Mass spectrometers have three main components: the ion source, the mass analyzer, and the detector, sometimes referred to as the electrometer. In MS, analytes are transferred into the gas phase, charged, filtered, and detected based on the mass-to-charge (m/z) ratio.
The ion source first generates gas-phase ions from the eluent stream and provides a focused ion beam to the mass analyzer. Next, the mass analyzer separates ions in time or space based on the respective m/z. Lastly, the detector converts the ions into time-based electrical signal and outputs a spectrum of the selected m/z within the scan range.
The key differentiator among mass spectrometers is the mass analyzer. Established examples include ion trap, quadrupole, time-of-flight, and orbitrap, with different analyzers providing advantages in resolution, speed, and sensitivity.
Tandem mass spectrometry (MS/MS) involves using multiple stages of mass analysis to gain more structural information and/or higher specificity than single-stage MS. In the first stage, precursor ions are isolated based on the m/z. Next, the selected ions are fragmented, often by acceleration through an inert gas. Finally, the fragmented ions are isolated and detected.
Explore our MS learning center to learn more about the benefits of mass detection.
FAQ
Most HPLC detectors work by converting a physiochemical property of an analyte into an electrical signal.
There are many varieties of HPLC detectors. The common ones used are UV-Vis, fluorescence, mass spectrometry, charged aerosol, evaporative light scattering, and refractive index detectors.
Contact us
* Required field