Search Thermo Fisher Scientific
Page Contents
Related Tables
Related Technical Notes
Get Chapter Downloads from The Molecular Probes Handbook, 11th edition |
Each of our amphiphilic probes comprises a moderately polar fluorescent dye with a lipophilic "tail." When used to stain membranes, including liposomes, the lipophilic portion of the probe tends to insert in the membrane and the polar fluorophore resides on the surface (Figure 13.2.1 in Fatty Acid Analogs and Phospholipids—Section 13.2), where it senses the membrane's surface environment and the surrounding medium.![]() Our lipophilic carbocyanines and styryl dyes (Dialkylcarbocyanine and Dialkylaminostyryl Probes—Section 13.4) are also amphiphilic molecules with a similar binding mode.
Our lipophilic carbocyanines and styryl dyes (Dialkylcarbocyanine and Dialkylaminostyryl Probes—Section 13.4) are also amphiphilic molecules with a similar binding mode.
This section includes the classic membrane probes DPH, TMA-DPH, ANS, bis-ANS, TNS, prodan, laurdan and nile red, and also some lipophilic BODIPY and Dapoxyl dyes developed in our laboratories. Although they bear little resemblance to natural products, these probes tend to localize within cell membranes or liposomes or at their aqueous interfaces, where they are often used to report on characteristics of their local environment, such as viscosity, polarity and lipid order.
Octadecyl Rhodamine B
The relief of the fluorescence self-quenching of octadecyl rhodamine B (O246) can be used to monitor membrane fusion ![]() —one of several experimental approaches developed for this application (Lipid-Mixing Assays of Membrane Fusion—Note 13.1). Octadecyl rhodamine B has been reported to undergo a potential-dependent "flip-flop" from one monolayer of a fluid-state phospholipid bilayer membrane to the other, with partial relief of its fluorescence quenching.
—one of several experimental approaches developed for this application (Lipid-Mixing Assays of Membrane Fusion—Note 13.1). Octadecyl rhodamine B has been reported to undergo a potential-dependent "flip-flop" from one monolayer of a fluid-state phospholipid bilayer membrane to the other, with partial relief of its fluorescence quenching.![]() Investigators have used octadecyl rhodamine B in conjunction with video microscopy
Investigators have used octadecyl rhodamine B in conjunction with video microscopy ![]() or digital imaging techniques
or digital imaging techniques ![]() to monitor viral fusion processes. Membrane fusion can also be followed by monitoring fluorescence resonance energy transfer to octadecyl rhodamine B from an acylaminofluorescein donor such as 5-hexadecanoylaminofluorescein.
to monitor viral fusion processes. Membrane fusion can also be followed by monitoring fluorescence resonance energy transfer to octadecyl rhodamine B from an acylaminofluorescein donor such as 5-hexadecanoylaminofluorescein.![]()
Fluorescence resonance energy transfer from fluorescein or dansyl labels to octadecyl rhodamine B has been used for structural studies of the blood coagulation factor IXa, EGF receptor and receptor-bound IgE.![]() Octadecyl rhodamine B has also been used to stain kinesin-generated membrane tubules,
Octadecyl rhodamine B has also been used to stain kinesin-generated membrane tubules,![]() to characterize detergent micelles,
to characterize detergent micelles,![]() to assay for lysosomal degradation of lipoproteins
to assay for lysosomal degradation of lipoproteins ![]() and to investigate the influence of proteins on lipid dynamics using time-resolved fluorescence anisotropy.
and to investigate the influence of proteins on lipid dynamics using time-resolved fluorescence anisotropy.![]()
Amphiphilic Fluoresceins
The amphiphilic fluorescein probes bind to membranes with the fluorophore at the aqueous interface and the alkyl tail protruding into the lipid interior. 5-Dodecanoylaminofluorescein is the hydrolysis product of our ImaGene Green C12-FDG β-galactosidase substrate (D2893, Detecting Glycosidases—Section 10.2). The homologous membrane probe 5-hexadecanoylaminofluorescein ![]() and the octadecyl ester of fluorescein
and the octadecyl ester of fluorescein ![]() have also been used as fluorescent labels.
have also been used as fluorescent labels.
Amphiphilic fluorescein probes are commonly used for fluorescence recovery after photobleaching (FRAP) measurements of lipid lateral diffusion.![]() Some researchers have reported that 5-hexadecanoylaminofluorescein stays predominantly in the outer membrane leaflet of epithelia and does not pass through tight junctions, whereas the dodecanoyl derivative can "flip-flop" to the inner leaflet at 20°C (but not at <10°C) and may also pass through tight junctions.
Some researchers have reported that 5-hexadecanoylaminofluorescein stays predominantly in the outer membrane leaflet of epithelia and does not pass through tight junctions, whereas the dodecanoyl derivative can "flip-flop" to the inner leaflet at 20°C (but not at <10°C) and may also pass through tight junctions.![]() More recent studies have indicated that the lack of tight junction penetration of 5-hexadecanoylaminofluorescein is due to probe aggregation rather than a significant difference in its transport properties.
More recent studies have indicated that the lack of tight junction penetration of 5-hexadecanoylaminofluorescein is due to probe aggregation rather than a significant difference in its transport properties.![]()
Diphenylhexatriene (DPH)
1,6-Diphenyl-1,3,5-hexatriene (DPH) continues to be a popular fluorescent probe of membrane interiors. We also offer the cationic DPH derivative TMA-DPH (see below). The orientation of DPH within lipid bilayers is loosely constrained. It is generally assumed to be oriented parallel to the lipid acyl chain axis (Figure 13.2.1A in Fatty Acid Analogs and Phospholipids—Section 13.2), but it can also reside in the center of the lipid bilayer parallel to the surface, as demonstrated by time-resolved fluorescence anisotropy and polarized fluorescence measurements of oriented samples.![]() DPH shows no partition preference between coexisting gel- and fluid-phase phospholipids.
DPH shows no partition preference between coexisting gel- and fluid-phase phospholipids.![]() Intercalation of DPH and its derivatives into membranes is accompanied by strong enhancement of their fluorescence; their fluorescence is practically negligible in water. The fluorescence decay of DPH in lipid bilayers is complex.
Intercalation of DPH and its derivatives into membranes is accompanied by strong enhancement of their fluorescence; their fluorescence is practically negligible in water. The fluorescence decay of DPH in lipid bilayers is complex.![]() Fluorescence decay data are often analyzed in terms of continuous lifetime distributions,
Fluorescence decay data are often analyzed in terms of continuous lifetime distributions,![]() which are in turn interpreted as being indicative of lipid environment heterogeneity.
which are in turn interpreted as being indicative of lipid environment heterogeneity.
DPH and its derivatives are cylindrically shaped molecules with absorption and fluorescence emission transition dipoles aligned approximately parallel to their long molecular axis. Consequently, their fluorescence polarization is high in the absence of rotational motion and is very sensitive to reorientation of the long axis resulting from interactions with surrounding lipids. These properties have led to their extensive use for membrane fluidity measurements.![]() The exact physical interpretation of these measurements has some contentious aspects. For instance, the probes are largely sensitive to only the angular reorientation of lipid acyl chains—a motion that does not necessarily correlate with other dynamic processes such as lateral diffusion.
The exact physical interpretation of these measurements has some contentious aspects. For instance, the probes are largely sensitive to only the angular reorientation of lipid acyl chains—a motion that does not necessarily correlate with other dynamic processes such as lateral diffusion.![]() Reviews on this subject
Reviews on this subject ![]() should be consulted for further discussion. Time-resolved fluorescence polarization measurements of lipid order are more physically rigorous because they allow the angular range of acyl chain reorientation ("lipid order") to be resolved from its rate, and considerable research has been devoted to the interpretation of these measurements.
should be consulted for further discussion. Time-resolved fluorescence polarization measurements of lipid order are more physically rigorous because they allow the angular range of acyl chain reorientation ("lipid order") to be resolved from its rate, and considerable research has been devoted to the interpretation of these measurements.![]()
TMA-DPH
Designed to improve the localization of DPH in the membrane, TMA-DPH (T204) contains a cationic trimethylammonium substituent that acts as a surface anchor.![]() Like DPH, this derivative readily partitions from aqueous dispersions into membranes and other lipid assemblies, accompanied by strong fluorescence enhancement. The lipid–water partition coefficient (Kp) for TMA-DPH (Kp = 2.4 × 105) is lower than for DPH (Kp = 1.3 × 106), reflecting the increased water solubility caused by the polar substituents.
Like DPH, this derivative readily partitions from aqueous dispersions into membranes and other lipid assemblies, accompanied by strong fluorescence enhancement. The lipid–water partition coefficient (Kp) for TMA-DPH (Kp = 2.4 × 105) is lower than for DPH (Kp = 1.3 × 106), reflecting the increased water solubility caused by the polar substituents.![]() The fluorescence decay lifetime of TMA-DPH is more sensitive to changes in lipid composition and temperature than is the fluorescence decay lifetime of DPH.
The fluorescence decay lifetime of TMA-DPH is more sensitive to changes in lipid composition and temperature than is the fluorescence decay lifetime of DPH.![]()
Staining of cell membranes by TMA-DPH is much more rapid than staining by DPH; however, the duration of plasma membrane surface staining by TMA-DPH before internalization into the cytoplasm is quite prolonged.![]() As a consequence, TMA-DPH introduced into Madin–Darby canine kidney (MDCK) cell plasma membranes does not diffuse through tight junctions and remains in the apical domain, whereas the anionic DPH propionic acid accumulates rapidly in intracellular membranes.
As a consequence, TMA-DPH introduced into Madin–Darby canine kidney (MDCK) cell plasma membranes does not diffuse through tight junctions and remains in the apical domain, whereas the anionic DPH propionic acid accumulates rapidly in intracellular membranes.![]() TMA-DPH residing in the plasma membrane can be extracted by washing with medium, thus providing a method for isolating internalized probe and monitoring endocytosis
TMA-DPH residing in the plasma membrane can be extracted by washing with medium, thus providing a method for isolating internalized probe and monitoring endocytosis ![]() (Probes for Following Receptor Binding and Phagocytosis—Section 16.1). Furthermore, because TMA-DPH is virtually nonfluorescent in water and binds in proportion to the available membrane surface,
(Probes for Following Receptor Binding and Phagocytosis—Section 16.1). Furthermore, because TMA-DPH is virtually nonfluorescent in water and binds in proportion to the available membrane surface,![]() its fluorescence intensity is sensitive to increases in plasma membrane surface area resulting from exocytosis.
its fluorescence intensity is sensitive to increases in plasma membrane surface area resulting from exocytosis.![]()
TMA-DPH fluorescence polarization measurements can be combined with video microscopy to provide spatially resolved images of phospholipid order in large liposomes and single cells.![]() Information regarding lipid order heterogeneity among cell populations can be obtained in a similar way using flow cytometry.
Information regarding lipid order heterogeneity among cell populations can be obtained in a similar way using flow cytometry.![]()
BODIPY Fluorophores
BODIPY fluorophore derivatives offer an unusual combination of nonpolar structure and long-wavelength absorption and fluorescence.![]() BODIPY dyes have small fluorescence Stokes shifts, extinction coefficients that are typically greater than 80,000 cm-1M-1 and high fluorescence quantum yields that are not diminished in water.
BODIPY dyes have small fluorescence Stokes shifts, extinction coefficients that are typically greater than 80,000 cm-1M-1 and high fluorescence quantum yields that are not diminished in water.![]() These dyes have applications as stains for neutral lipids and as tracers for oils and other nonpolar liquids. In addition, their photostability is generally high; this, together with other favorable characteristics (very low triplet–triplet absorption), make the BODIPY 493/503 and BODIPY 505/515 fluorophores excellent choices for flashlamp-pumped laser dyes.
These dyes have applications as stains for neutral lipids and as tracers for oils and other nonpolar liquids. In addition, their photostability is generally high; this, together with other favorable characteristics (very low triplet–triplet absorption), make the BODIPY 493/503 and BODIPY 505/515 fluorophores excellent choices for flashlamp-pumped laser dyes.![]()
Staining with the BODIPY 493/503 dye (D3922) has been shown by flow cytometry to be more specific for cellular lipid droplets than staining with nile red ![]() (N1142). The low molecular weight of the BODIPY 493/503 dye (262 daltons) results in the probe having a relatively fast diffusion rate in membranes.
(N1142). The low molecular weight of the BODIPY 493/503 dye (262 daltons) results in the probe having a relatively fast diffusion rate in membranes.![]() The BODIPY 493/503 dye has also been used to detect neutral compounds in a microchip channel separation device.
The BODIPY 493/503 dye has also been used to detect neutral compounds in a microchip channel separation device.![]()
BODIPY 505/515 (D3921) rapidly permeates cell membranes of live zebrafish embryos,![]() selectively staining cytoplasmic yolk platelets. This staining provides dramatic contrast enhancement of cytoplasm relative to nucleoplasm and interstitial spaces, allowing individual cell boundaries and cell nuclei to be imaged clearly with a confocal laser-scanning microscope (
selectively staining cytoplasmic yolk platelets. This staining provides dramatic contrast enhancement of cytoplasm relative to nucleoplasm and interstitial spaces, allowing individual cell boundaries and cell nuclei to be imaged clearly with a confocal laser-scanning microscope (![]() ).
).
The very long–wavelength BODIPY 665/676 dye (B3932) has fluorescence that is not visible to the human eye; however, it has found use as a probe for reactive oxygen species ![]() (Generating and Detecting Reactive Oxygen Species—Section 18.2).
(Generating and Detecting Reactive Oxygen Species—Section 18.2).
BODIPY FL C5-Ceramide
BODIPY FL C5-ceramide (D3521, B22650; Sphingolipids, Steroids, Lipopolysaccharides and Related Probes—Section 13.3) stains the plasma membrane, Golgi apparatus and cytoplasmic particles within the superficial enveloping layer (EVL) of the embryos. Once the fluorescent lipid percolates through the EVL epithelium, however, it remains localized within the interstitial fluid of the embryo and freely diffuses between cells (![]() ). Vital staining with BODIPY FL C5-ceramide thus allows hundreds of cells to be imaged en masse during morphogenetic movements.
). Vital staining with BODIPY FL C5-ceramide thus allows hundreds of cells to be imaged en masse during morphogenetic movements.![]()
CellTrace BODIPY TR Methyl Ester
Many research and biotechnological applications require detailed three- and four-dimensional visualization of embryonic cells labeled with green-fluorescent protein (GFP) within their native tissue environments. Fluorescent counterstains that label all the cells in a living embryo provide a histological context for the GFP-expressing cells in the specimen. The red-fluorescent CellTrace BODIPY TR methyl ester (C34556) is an excellent counterstain for cells and tissues that are expressing GFP.![]() This dye readily permeates cell membranes and selectively stains mitochondria and endomembranous organelles such as endoplasmic reticulum and the Golgi apparatus, but does not appear to localize in the plasma membrane. These localization properties make the dye an ideal vital stain that can be used to reveal: (1) the location and shapes of cell nuclei, (2) the shapes of cells within embryonic tissues and (3) the boundaries of organ-forming tissues within the whole embryo.
This dye readily permeates cell membranes and selectively stains mitochondria and endomembranous organelles such as endoplasmic reticulum and the Golgi apparatus, but does not appear to localize in the plasma membrane. These localization properties make the dye an ideal vital stain that can be used to reveal: (1) the location and shapes of cell nuclei, (2) the shapes of cells within embryonic tissues and (3) the boundaries of organ-forming tissues within the whole embryo.![]() Furthermore, CellTrace BODIPY TR methyl ester staining is retained after formaldehyde fixation and permeabilization with Triton X-100, and the dye does not appear to produce any teratogenic effects on embryonic development. The emission spectra of enhanced GFP (EGFP) and CellTrace BODIPY TR methyl ester are well separated, with peaks at 508 nm and 625 nm, respectively (Figure 13.5.1), allowing simultaneous dual-channel confocal imaging without significant overspill of GFP fluorescence into the CellTrace BODIPY TR methyl ester detection channel.
Furthermore, CellTrace BODIPY TR methyl ester staining is retained after formaldehyde fixation and permeabilization with Triton X-100, and the dye does not appear to produce any teratogenic effects on embryonic development. The emission spectra of enhanced GFP (EGFP) and CellTrace BODIPY TR methyl ester are well separated, with peaks at 508 nm and 625 nm, respectively (Figure 13.5.1), allowing simultaneous dual-channel confocal imaging without significant overspill of GFP fluorescence into the CellTrace BODIPY TR methyl ester detection channel.
The Image-iT LIVE Intracellular Membrane and Nuclear Labeling Kit (I34407, Tracers for Membrane Labeling—Section 14.4) provides the red-fluorescent CellTrace BODIPY TR methyl ester along with the blue-fluorescent Hoechst 33342 dye for highly selective staining of the intracellular membranes and nuclei, respectively, of live or fixed cells or tissues (![]() ). These two fluorescent stains were especially chosen for their compatibility with live GFP-expressing cells, and they can be combined into one staining solution to save labeling time and wash steps while still providing optimal staining.
). These two fluorescent stains were especially chosen for their compatibility with live GFP-expressing cells, and they can be combined into one staining solution to save labeling time and wash steps while still providing optimal staining.
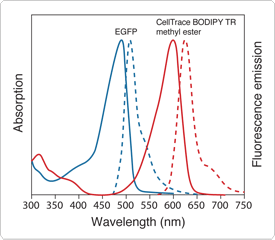
Figure 13.5.1 Normalized absorption (—) and fluorescence emission (---) spectra of enhanced green-fluorescent protein (EGFP) and CellTrace BODIPY TR methyl ester (C34556).
Nonpolar Pyrene Probe
1,3-Bis-(1-pyrene)propane has two pyrene moieties linked by a three-carbon alkylene spacer. This probe is somewhat analogous to the bis-pyrenyl phospholipids (Fatty Acid Analogs and Phospholipids—Section 13.2) in that excimer formation (and, consequently, the fluorescence emission wavelength) is controlled by intramolecular rather than bimolecular interactions. Thus, this probe is highly sensitive to constraints imposed by its environment, and can therefore be used as a viscosity sensor for interior regions of lipoproteins, membranes, micelles, liquid crystals and synthetic polymers.![]() Because excimer formation results in a spectral shift (Figure 13.5.2), the probe may be useful for ratio imaging of molecular mobility.
Because excimer formation results in a spectral shift (Figure 13.5.2), the probe may be useful for ratio imaging of molecular mobility.![]() However, pyrene fatty acids (Fatty Acid Analogs and Phospholipids—Section 13.2) appear to be preferable for this purpose because the uptake of 1,3-bis-(1-pyrene)propane by cells is limited.
However, pyrene fatty acids (Fatty Acid Analogs and Phospholipids—Section 13.2) appear to be preferable for this purpose because the uptake of 1,3-bis-(1-pyrene)propane by cells is limited.
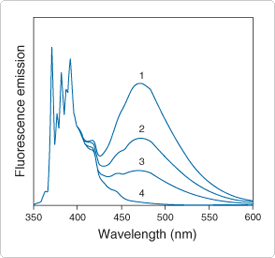
Figure 13.5.2 Excimer formation by pyrene in ethanol. Spectra are normalized to the 371.5 nm peak of the monomer. All spectra are essentially identical below 400 nm after normalization. Spectra are as follows: 1) 2 mM pyrene, purged with argon to remove oxygen; 2) 2 mM pyrene, air-equilibrated; 3) 0.5 mM pyrene (argon-purged); and 4) 2 µM pyrene (argon-purged). The monomer-to-excimer ratio (371.5 nm/470 nm) is dependent on both pyrene concentration and the excited-state lifetime, which is variable because of quenching by oxygen.
Nile Red
The phenoxazine dye nile red (N1142) is used to localize and quantitate lipids, particularly neutral lipid droplets within cells.![]() It is selective for neutral lipids such as cholesteryl esters
It is selective for neutral lipids such as cholesteryl esters ![]() (and also, therefore, for lipoproteins) and is suitable for staining lysosomal phospholipid inclusions.
(and also, therefore, for lipoproteins) and is suitable for staining lysosomal phospholipid inclusions.![]() Nile red is almost nonfluorescent in water and other polar solvents but undergoes fluorescence enhancement and large absorption and emission blue shifts in nonpolar environments.
Nile red is almost nonfluorescent in water and other polar solvents but undergoes fluorescence enhancement and large absorption and emission blue shifts in nonpolar environments.![]() Its fluorescence enhancement upon binding to proteins is weaker than that produced by its association with lipids
Its fluorescence enhancement upon binding to proteins is weaker than that produced by its association with lipids ![]() (Figure 13.5.3). Ligand-binding studies on tubulin and tryptophan synthase
(Figure 13.5.3). Ligand-binding studies on tubulin and tryptophan synthase ![]() have exploited the environmental sensitivity of nile red's fluorescence. Nile red has also been used to detect sphingolipids on thin-layer chromatograms
have exploited the environmental sensitivity of nile red's fluorescence. Nile red has also been used to detect sphingolipids on thin-layer chromatograms ![]() and to stain proteins after SDS-polyacrylamide gel electrophoresis.
and to stain proteins after SDS-polyacrylamide gel electrophoresis.![]()
Bimane Azide
Bimane azide is a small blue-fluorescent photoreactive alkyl azide (excitation/emission maxima ~375/458 nm) for photoaffinity labeling of proteins, potentially including membrane proteins from within the cell membrane. This reactive fluorophore's small size may reduce the likelihood that the label will interfere with the function of the biomolecule, an important advantage for site-selective probes.
Steatosis, the intracellular accumulation of neutral lipids as lipid droplets or globules, is often triggered by drugs that affect the metabolism of fatty acids or neutral lipids. LipidTOX neutral lipid stains were developed to characterize the effects of drugs and other compounds on lipid metabolism in mammalian cell lines. LipidTOX neutral lipid stains have an extremely high affinity for neutral lipid droplets. These reagents are added after cell fixation and do not require subsequent wash steps after incubation with the sample. Key advantages of this series of neutral lipid stains over conventional stains such as Nile Red include their ready-to-use formulations, their flexibility for multiplexing protocols and their compatibility with LipidTOX phospholipid stains (H34350, H34351; Fatty Acid Analogs and Phospholipids—Section 13.2).
LipidTOX neutral lipid stains are available with green, red and deep red fluorescence emission:
- HCS LipidTOX Green neutral lipid stain (H34475), with excitation/emission maxima ~495/505 nm (Figure 13.5.4)
- HCS LipidTOX Red neutral lipid stain (H34476), with excitation/emission maxima ~577/609 nm
- HCS LipidTOX Deep Red neutral lipid stain (H34477), with excitation/emission maxima ~637/655 nm
These HCS LipidTOX neutral lipid stains have been used to image intracellular lipid accumulation in rat cortical neurons, COS-7 cells and hepatitis C virus (HCV)–infected FT3-7 human hepatoma cells.![]() HCS LipidTOX Red neutral lipid stain was used to detect RNAi knockdown of acyl-coenzyme A:cholesterol acyl transferase, isoform 1 (ACAT-1), an endoplasmic reticulum enzyme that regulates the equilibrium between free cholesterol and cholesteryl esters in cells.
HCS LipidTOX Red neutral lipid stain was used to detect RNAi knockdown of acyl-coenzyme A:cholesterol acyl transferase, isoform 1 (ACAT-1), an endoplasmic reticulum enzyme that regulates the equilibrium between free cholesterol and cholesteryl esters in cells.![]()
LipidTOX Green neutral lipid stain is also a component of the HCS LipidTOX Phospholipidosis and Steatosis Detection Kit (H34158, Fatty Acid Analogs and Phospholipids—Section 13.2), which provides a complete set of reagents for performing high-content screening (HCS) assays to detect and distinguish the intracellular accumulation of phospholipids (phospholipidosis) and of neutral lipids (steatosis) in mammalian cell lines after exposure to test compounds. In addition, HCS LipidTOX neutral lipid stains can be used to monitor the formation and differentiation of adipocytes, a process called adipogenesis. Adipogenesis is of acute interest to the biomedical and drug discovery community as it plays an important role in diseases such as obesity, diabetes and atherosclerosis.
HCS LipidTOX neutral lipid stains are designed for fixed–end point workflows in which formaldehyde-fixed cells in microplates are processed, imaged and analyzed. These stains can easily be detected with fluorescence microscopes or HCS readers equipped with standard filter sets.
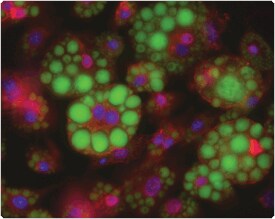
Figure 13.5.4 (FABP4) antibody labeling in adipocytes. Adipocytes differentiated from 3T3-L1 mouse fibroblasts were fixed with formaldehyde and permeabilized with saponin before labeling with rabbit anti–fatty acid binding protein (FABP4) IgG (red). These cells were then stained with LipidTOX Green neutral lipid stain (H34475, green), counterstained with DAPI (D1306, D21490; blue) and mounted in ProLong Gold antifade reagent (P36930).
Prodan and Laurdan
Prodan, introduced by Weber and Farris in 1979, has both electron-donor and electron-acceptor substituent, resulting in a large excited-state dipole moment and extensive solvent polarity–dependent fluorescence shifts ![]() (Figure 13.5.5). Several variants of the original probe have since been prepared, including the lipophilic derivative laurdan (D250) and thiol-reactive derivatives acrylodan and badan (A433, B6057; Thiol-Reactive Probes Excited with Ultraviolet Light—Section 2.3), which can be used to confer the environment-sensitive properties of this fluorophore on bioconjugates.
(Figure 13.5.5). Several variants of the original probe have since been prepared, including the lipophilic derivative laurdan (D250) and thiol-reactive derivatives acrylodan and badan (A433, B6057; Thiol-Reactive Probes Excited with Ultraviolet Light—Section 2.3), which can be used to confer the environment-sensitive properties of this fluorophore on bioconjugates.
When prodan or its derivatives are incorporated into membranes, their fluorescence spectra are sensitive to the physical state of the surrounding phospholipids.![]() In membranes, prodan appears to localize at the surface,
In membranes, prodan appears to localize at the surface,![]() although Fourier transform infrared (FTIR) measurements indicate some degree of penetration into the lipid interior.
although Fourier transform infrared (FTIR) measurements indicate some degree of penetration into the lipid interior.![]() Excited-state relaxation of prodan is sensitive to the nature of the linkage (ester or ether) between phospholipid hydrocarbon tails and the glycerol backbone.
Excited-state relaxation of prodan is sensitive to the nature of the linkage (ester or ether) between phospholipid hydrocarbon tails and the glycerol backbone.![]() In contrast, laurdan's excited-state relaxation is independent of head-group type, and is instead determined by water penetration into the lipid bilayer.
In contrast, laurdan's excited-state relaxation is independent of head-group type, and is instead determined by water penetration into the lipid bilayer.![]() Two-photon infrared excitation techniques have been successfully applied to both prodan and laurdan, although both probes nominally require ultraviolet excitation
Two-photon infrared excitation techniques have been successfully applied to both prodan and laurdan, although both probes nominally require ultraviolet excitation ![]() (~360 nm).
(~360 nm).
Much experimental work using these probes has sought to characterize coexisting lipid domains based on their distinctive fluorescence spectra,![]() an approach that is intrinsically amenable to dual-wavelength ratio measurements.
an approach that is intrinsically amenable to dual-wavelength ratio measurements.![]() Other applications include detecting nonbilayer lipid phases,
Other applications include detecting nonbilayer lipid phases,![]() mapping changes in membrane structure induced by cholesterol and alcohols
mapping changes in membrane structure induced by cholesterol and alcohols ![]() and assessing the polarity of lipid/water interfaces.
and assessing the polarity of lipid/water interfaces.![]() Like ANS (see below), prodan is also useful as a noncovalently interacting probe for proteins.
Like ANS (see below), prodan is also useful as a noncovalently interacting probe for proteins.![]()
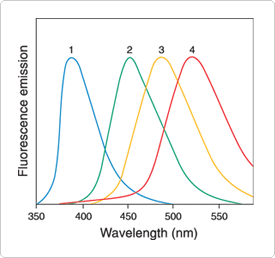
Figure 13.5.5 Normalized emission spectra of prodan excited at 345 nm in 1) cyclohexane, 2) dimethylformamide, 3) ethanol and 4) water.
Dapoxyl Derivative
We have developed a variety of probes based on our Dapoxyl fluorophore.![]() Dapoxyl sulfonamide derivatives exhibit UV absorption with maxima near 370 nm, extinction coefficients >24,000 cm-1M-1 and Stokes shifts in excess of 200 nm. Dapoxyl sulfonic acid is an amphiphilic Dapoxyl derivative with generally similar properties and applications to anilinonaphthalene sulfonate (ANS) (Monitoring Protein-Folding Processes with Environment-Sensitive Dyes—Note 9.1). Both ANS and Dapoxyl sulfonic acid have been used in a drug-discovery assay based on the detection of protein thermal denaturation shifts.
Dapoxyl sulfonamide derivatives exhibit UV absorption with maxima near 370 nm, extinction coefficients >24,000 cm-1M-1 and Stokes shifts in excess of 200 nm. Dapoxyl sulfonic acid is an amphiphilic Dapoxyl derivative with generally similar properties and applications to anilinonaphthalene sulfonate (ANS) (Monitoring Protein-Folding Processes with Environment-Sensitive Dyes—Note 9.1). Both ANS and Dapoxyl sulfonic acid have been used in a drug-discovery assay based on the detection of protein thermal denaturation shifts.![]() Reactive versions of the Dapoxyl fluorophore are described in Coumarins, Pyrenes and Other Ultraviolet Light-Excitable Fluorophores—Section 1.7 and Derivatization Reagents for Carboxylic Acids and Carboxamides—Section 3.4.
Reactive versions of the Dapoxyl fluorophore are described in Coumarins, Pyrenes and Other Ultraviolet Light-Excitable Fluorophores—Section 1.7 and Derivatization Reagents for Carboxylic Acids and Carboxamides—Section 3.4.
Anilinonaphthalenesulfonate (ANS) and Related Derivatives
The use of anilinonaphthalene sulfonates (ANS) as fluorescent probes dates back to the pioneering work of Weber in the 1950s, and this class of probes remains valuable for studying both membrane surfaces and proteins. Slavik's 1982 review of its properties is recommended reading, especially for the extensive compilation of spectral data.![]() The primary member of this class, 1,8-ANS (A47), and its analogs 2,6-ANS and 2,6-TNS are all essentially nonfluorescent in water, only becoming appreciably fluorescent when bound to membranes (quantum yields ~0.25) or proteins (quantum yields ~0.7)
The primary member of this class, 1,8-ANS (A47), and its analogs 2,6-ANS and 2,6-TNS are all essentially nonfluorescent in water, only becoming appreciably fluorescent when bound to membranes (quantum yields ~0.25) or proteins (quantum yields ~0.7) ![]() (Figure 13.5.3). This property makes them sensitive indicators of protein folding, conformational changes
(Figure 13.5.3). This property makes them sensitive indicators of protein folding, conformational changes ![]() and other processes that modify the exposure of the probe to water (Monitoring Protein-Folding Processes with Environment-Sensitive Dyes—Note 9.1). Fluorescence of 2,6-ANS is also enhanced by cyclodextrins, permitting a sensitive method for separating and analyzing cyclodextrins with capillary electrophoresis.
and other processes that modify the exposure of the probe to water (Monitoring Protein-Folding Processes with Environment-Sensitive Dyes—Note 9.1). Fluorescence of 2,6-ANS is also enhanced by cyclodextrins, permitting a sensitive method for separating and analyzing cyclodextrins with capillary electrophoresis.![]()
Bis-ANS
Bis-ANS (B153) is superior to 1,8-ANS as a probe for nonpolar cavities in proteins, often binding with an affinity that is orders-of-magnitude higher.![]() Bis-ANS has particularly high affinity for nucleotide-binding sites of some proteins.
Bis-ANS has particularly high affinity for nucleotide-binding sites of some proteins.![]() It is also useful as a structural probe for tubulin
It is also useful as a structural probe for tubulin ![]() and as an inhibitor of microtubule assembly.
and as an inhibitor of microtubule assembly.![]() Covalent photoincorporation of bis-ANS into proteins has been reported.
Covalent photoincorporation of bis-ANS into proteins has been reported.![]()
DCVJ
The styrene derivative DCVJ is a sensitive indicator of tubulin assembly and actin polymerization.![]() The fluorescence quantum yield of DCVJ is strongly dependent on environmental rigidity, resulting in large fluorescence increases when the dye binds to antibodies
The fluorescence quantum yield of DCVJ is strongly dependent on environmental rigidity, resulting in large fluorescence increases when the dye binds to antibodies ![]() and when it is compressed in synthetic polymers or phospholipid membrane interiors.
and when it is compressed in synthetic polymers or phospholipid membrane interiors.![]() DCVJ has been used for microviscosity measurements of phospholipid bilayers.
DCVJ has been used for microviscosity measurements of phospholipid bilayers.![]()
For a detailed explanation of column headings, see Definitions of Data Table Contents
| Cat # | MW | Storage | Soluble | Abs | EC | Em | Solvent | Notes |
|---|---|---|---|---|---|---|---|---|
| A47 1,8-ANS | 299.34 | L | pH >6, DMF | 372 | 7800 | 480 | MeOH | 1 |
| 2,6-ANS | 299.34 | L | DMF | 319 | 27,000 | 422 | MeOH | 1 |
| B153 Bis-ANS | 672.85 | L | pH >6 | 395 | 23,000 | 500 | MeOH | 1, 2 |
| 1,3-bis-(1-pyrenyl)propane | 444.57 | L | MeCN, CHCl3 | 344 | 80,000 | 378 | MeOH | 3 |
| B3932 BODIPY 665/676 (lipid peroxidation sensor) | 448.32 | F,L | DMSO, CHCl3 | 665 | 161,000 | 676 | MeOH | 4 |
| bimane azide | 233.23 | F,D,L | DMSO | 375 | 6000 | 458 | MeOH | |
| C34556 CellTracker BODIPY TR methyl ester (lipophilic counterstain for GFP) | 438.25 | F,D,L | DMSO | 588 | 68,000 | 616 | MeOH | 5 |
| 5-dodecanoylaminofluorescein | 529.63 | L | DMSO, EtOH | 495 | 85,000 | 518 | MeOH | 6 |
| DPH | 232.32 | L | DMF, MeCN | 350 | 88,000 | 452 | MeOH | 7, 8 |
| D250 laurdan | 353.55 | L | DMF, MeCN | 364 | 20,000 | 497 | MeOH | 9 |
| D3921 BODIPY 505/515 | 248.08 | F,L | EtOH, DMSO | 502 | 98,000 | 510 | MeOH | 4 |
| D3922 BODIPY 493/503 | 262.11 | F,L | EtOH, DMSO | 493 | 89,000 | 504 | MeOH | 4 |
| DCVJ | 249.31 | L | DMF, DMSO | 456 | 61,000 | 493 | MeOH | |
| Dapoxyl sulfonic acid | 366.37 | L | DMSO, H2O | 358 | 25,000 | 517 | MeOH | 10 |
| fluorescein octadecyl ester | 584.79 | L | DMSO, EtOH | 504 | 95,000 | 525 | MeOH | 6 |
| 5-hexadecanoylaminofluorescein | 585.74 | L | DMSO, EtOH | 497 | 92,000 | 519 | MeOH | 6 |
| H34475 HCS LipidTOX Green Neutral Lipid Stain | ~300 | F,L | DMSO | 495 | 94,000 | 505 | MeOH | 5, 13 |
| H34476 HCS LipidTOX Red Neutral Lipid Stain | ~400 | F,L | DMSO | 574 | 62,000 | 609 | MeOH | 5, 13 |
| H34477 HCS LipidTOX Deep Red Neutral Lipid Stain | ~350 | F,L | DMSO | 626 | 68,000 | 648 | MeOH | 5, 13 |
| N1142 nile red | 318.37 | L | DMF, DMSO | 552 | 45,000 | 636 | MeOH | 11 |
| O246 octadecyl rhodamine B (R18) | 731.50 | F,DD,L | DMSO, EtOH | 556 | 125,000 | 578 | MeOH | 12 |
| prodan | 227.31 | L | DMF, MeCN | 363 | 19,000 | 497 | MeOH | 9 |
| 2,6-TNS | 335.35 | L | DMF | 318 | 26,000 | 443 | MeOH | 1 |
| T204 TMA-DPH | 461.62 | D,L | DMF, DMSO | 355 | 75,000 | 430 | MeOH | 7 |
| ||||||||
For Research Use Only. Not for use in diagnostic procedures.
