Search Thermo Fisher Scientific
Page Contents
- Viability/Cytotoxicity Assays Using Esterase Substrates
- Viability/Cytotoxicity and Gram Stain Assays Using Nucleic Acid Stains
- Viability/Cytotoxicity Assays That Measure Oxidation or Reduction
- Other Viability/Cytotoxicity Assay Methods
- Fluorescent Antibiotics and Related Probes
- Data Table
- Ordering Information
Fluorometric assays of cell viability and cytotoxicity are easy to perform with the use of a fluorescence microscope, fluorometer, fluorescence microplate reader or flow cytometer,![]() and they offer many advantages over traditional colorimetric and radioactivity-based assays. This section describes our numerous reagents for conducting viability and cytotoxicity assays in a wide variety of cells, including those of animal origin as well as bacteria and yeast. Following this discussion of individual reagents is Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3, which contains a thorough description of each of our viability and cytotoxicity kits, including the:
and they offer many advantages over traditional colorimetric and radioactivity-based assays. This section describes our numerous reagents for conducting viability and cytotoxicity assays in a wide variety of cells, including those of animal origin as well as bacteria and yeast. Following this discussion of individual reagents is Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3, which contains a thorough description of each of our viability and cytotoxicity kits, including the:
- LIVE/DEAD Viability/Cytotoxicity Kit (L3224)
- LIVE/DEAD Reduced Biohazard Cell Viability Kit (L7013)
- LIVE/DEAD Fixable Dead Cell Stain Kits (8 different fluorescent stain kits and the Sampler Kit L34960)
- LIVE/DEAD Cell-Mediated Cytotoxicity Kit (L7010)
- LIVE/DEAD Sperm Viability Kit (L7011)
- LIVE/DEAD Cell Vitality Assay Kit (L34951)
- LIVE/DEAD Violet Viability/Vitality Kit (L34958)
- Vybrant Cell Metabolic Assay Kit *with C12-resazurin* (V23110)
- Vybrant Cytotoxicity Assay Kit *G6PD release assay* (V23111)
- LIVE/DEAD Yeast Viability Kit (L7009)
- LIVE/DEAD FungaLight Yeast Viability Kit (L34952)
- FungaLight CFDA AM/Propidium Iodide Yeast Vitality Kit (F34953)
- LIVE/DEAD BacLight Bacterial Viability Kits (L7007, L7012, L13152, L34856)
- BacLight RedoxSensor Vitality Kits (B34954, B34956)
- BacLight Bacterial Membrane Potential Kit (B34950)
- ViaGram Red+ Bacterial Gram Stain and Viability Kit (V7023)
Also discussed both in this section and Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3 are our unique single-step reagents and kits for assessing gram sign and for simultaneously determining gram sign and viability of bacteria, as well as our novel fluorescent antibiotics. Assays for Cell Enumeration, Cell Proliferation and Cell Cycle—Section 15.4 describes our important probes for quantitating cell proliferation, analyzing the cell cycle and detecting the presence of bacteria and mycoplasma.
We prepare a wide variety of fluorogenic esterase substrates—including calcein AM, BCECF AM and various fluorescein diacetate derivatives—that can be passively loaded into adherent and nonadherent cells. These cell-permeant esterase substrates serve as viability probes that measure both enzymatic activity, which is required to activate their fluorescence, and cell-membrane integrity, which is required for intracellular retention of their fluorescent products.
As electrically neutral or near-neutral molecules, the esterase substrates freely diffuse into most cells. In general, cell loading of acetate or acetoxymethyl ester derivatives is accomplished by initially preparing a 1–10 mM stock solution of the dye in dimethylsulfoxide (DMSO) and then diluting the stock solution into the cell medium to a final concentration of 1–25 µM (Loading and Calibration of Intracellular Ion Indicators—Note 19.1). Once inside the cell, these nonfluorescent substrates are converted by nonspecific intracellular esterases into fluorescent products that are retained by cells with intact plasma membranes. In contrast, both the unhydrolyzed substrates and their products rapidly leak from dead or damaged cells with compromised membranes, even when the cells retain some residual esterase activity. Low incubation temperatures and highly charged esterase products usually favor retention, although the rate of dye loss from viable cells also depends to a large extent on cell type (see "Multidrug Resistance" in Probes for Cell Adhesion, Chemotaxis, Multidrug Resistance and Glutathione—Section 15.6). For example, mast cells and epithelial cells actively secrete many polar products.![]() Esterase substrates for cell viability studies—Table 15.1 lists Molecular Probes esterase substrates that have been used for cell viability studies and compares their cell loading, retention and pH sensitivity. Many of the applications of these esterase substrates—for example, viability, cytotoxicity and adhesion assays—closely parallel those of 51Cr-release assays, except that the fluorescent probes do not carry the risks or the disposal costs associated with the use of radioactive materials.
Esterase substrates for cell viability studies—Table 15.1 lists Molecular Probes esterase substrates that have been used for cell viability studies and compares their cell loading, retention and pH sensitivity. Many of the applications of these esterase substrates—for example, viability, cytotoxicity and adhesion assays—closely parallel those of 51Cr-release assays, except that the fluorescent probes do not carry the risks or the disposal costs associated with the use of radioactive materials.
CellTrace Calceins: Calcein AM, Calcein Blue AM, Calcein Violet AM and Calcein Red-Orange AM
Of the dyes listed in Esterase substrates for cell viability studies—Table 15.1, calcein AM (C1430, C3099, C3100MP) stands out as the premier indicator of cell viability due to its superior cell retention and the relative insensitivity of its fluorescence to pH in the physiological range.![]() Calcein AM, also called CellTrace calcein green AM (C34852), is a widely used probe for assays of cell adhesion, chemotaxis and multidrug resistance (Probes for Cell Adhesion, Chemotaxis, Multidrug Resistance and Glutathione—Section 15.6). Calcein (C481, Polar Tracers—Section 14.3), which is the hydrolysis product of calcein AM, is a polyanionic fluorescein derivative that has about six negative charges and two positive charges at pH 7.
Calcein AM, also called CellTrace calcein green AM (C34852), is a widely used probe for assays of cell adhesion, chemotaxis and multidrug resistance (Probes for Cell Adhesion, Chemotaxis, Multidrug Resistance and Glutathione—Section 15.6). Calcein (C481, Polar Tracers—Section 14.3), which is the hydrolysis product of calcein AM, is a polyanionic fluorescein derivative that has about six negative charges and two positive charges at pH 7.![]() Calcein is better retained in viable cells than are fluorescein, carboxyfluorescein and BCECF (Figure 15.2.1) and tends to have brighter fluorescence in a number of mammalian cell types. Calcein AM has the ability to penetrate intact cornea, revealing cell viability, morphology and organization of living cornea.
Calcein is better retained in viable cells than are fluorescein, carboxyfluorescein and BCECF (Figure 15.2.1) and tends to have brighter fluorescence in a number of mammalian cell types. Calcein AM has the ability to penetrate intact cornea, revealing cell viability, morphology and organization of living cornea.![]() Furthermore, unlike some other dyes—including BCECF AM—calcein AM does not interfere with leukocyte chemotaxis or superoxide production, nor does it affect lymphocyte–target cell conjugation.
Furthermore, unlike some other dyes—including BCECF AM—calcein AM does not interfere with leukocyte chemotaxis or superoxide production, nor does it affect lymphocyte–target cell conjugation.![]() Leakage of calcein from calcein AM–loaded cells has been used to measure the increase in membrane permeability that occurs above physiological temperatures,
Leakage of calcein from calcein AM–loaded cells has been used to measure the increase in membrane permeability that occurs above physiological temperatures,![]() as well as to assay for cytotoxic T lymphocyte activity.
as well as to assay for cytotoxic T lymphocyte activity.![]() Fluorescence of extracellular calcein that has leaked from cells or that has been lost during secretion, lysis or ATP-dependent anion transport can be quenched by 5 µM Co2+ ion or by Mn2+ ion. Heavy-atom quenching of calcein provides a means of detecting dye leakage and quantitating only the intracellular fluorescence.
Fluorescence of extracellular calcein that has leaked from cells or that has been lost during secretion, lysis or ATP-dependent anion transport can be quenched by 5 µM Co2+ ion or by Mn2+ ion. Heavy-atom quenching of calcein provides a means of detecting dye leakage and quantitating only the intracellular fluorescence.![]()
Dihydrocalcein AM is a reasonably stable, chemically reduced form of calcein AM that requires both hydrolysis by intracellular esterases and oxidation within the cell to produce the green-fluorescent calcein dye. Dihydrocalcein AM resembles 2',7'-dichlorodihydrofluorescein diacetate (D399), the important indicator for oxidative activity in cells (see below), except that its oxidation product (calcein) should be better retained in cells than is the oxidation product of 2',7'-dichlorodihydrofluorescein diacetate. Dihydrocalcein AM (D23805) is available as a set of 20 vials, each containing 50 µg of the product.
Calcein blue AM, also called CellTrace calcein blue AM (C1429, C34853), is a viability indicator for use with instruments optimized for the detection of blue fluorescence.![]() This tracer possesses AM esters that allow its passive diffusion across cell membranes. Before de-esterification, CellTrace calcein blue AM is only weakly fluorescent (excitation/emission maxima ~322/435 nm). Upon cleavage of the AM esters by intracellular esterases, however, this tracer becomes relatively polar and is retained by cells for several hours. In addition, its fluorescence intensity increases and its fluorescence spectra shifts to longer wavelengths, with excitation/emission maxima of ~360/449 nm. Calcein blue AM is useful for viability measurements in combination with our SYTOX Green nucleic acid stain (see below) and other green- or red-fluorescent probes.
This tracer possesses AM esters that allow its passive diffusion across cell membranes. Before de-esterification, CellTrace calcein blue AM is only weakly fluorescent (excitation/emission maxima ~322/435 nm). Upon cleavage of the AM esters by intracellular esterases, however, this tracer becomes relatively polar and is retained by cells for several hours. In addition, its fluorescence intensity increases and its fluorescence spectra shifts to longer wavelengths, with excitation/emission maxima of ~360/449 nm. Calcein blue AM is useful for viability measurements in combination with our SYTOX Green nucleic acid stain (see below) and other green- or red-fluorescent probes.
Calcein violet AM (C34858) is optimized for use in flow cytometry. The enzymatic conversion of the virtually nonfluorescent cell-permeant calcein violet AM to the intensely fluorescent calcein violet (excitation/emission maxima ~400/452 nm) is efficiently excited by the 405 nm violet diode laser.
We also offer CellTrace calcein red-orange AM (C34851). Upon cleavage by intracellular esterases, CellTrace calcein red-orange (excitation/emission maxima ~576/589 nm) is well retained by live cells that possess intact plasma membranes. Unlike calcein AM, CellTrace calcein red-orange AM is intrinsically fluorescent; thus, an additional wash step may be necessary to minimize background fluorescence from dye that is not taken up by cells. CellTrace calcein red-orange AM is a useful cell tracer and indicator of cell viability and can be used in combination with green-fluorescent probes, such as the fluo-4 Ca2+ indicator ![]() (Fluorescent Ca2+ Indicators Excited with Visible Light—Section 19.3).
(Fluorescent Ca2+ Indicators Excited with Visible Light—Section 19.3).
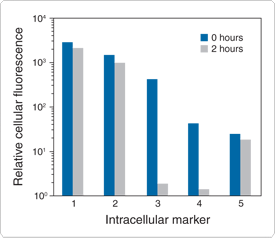
Figure 15.2.1 Loading and retention characteristics of intracellular marker dyes. Cells of a human lymphoid line (GePa) were loaded with the following cell-permeant acetoxymethyl ester (AM) or acetate derivatives of fluorescein: 1) calcein AM (C1430, C3099, C3100MP), 2) BCECF AM (B1150), 3) fluorescein diacetate (FDA,F1303), 4) carboxyfluorescein diacetate (CFDA, C1354) and 5) CellTracker Green CMFDA (5-chloromethylfluorescein diacetate, C2925, C7025). Cells were incubated in 4 µM staining solutions in Dulbecco's modified eagle medium containing 10% fetal bovine serum (DMEM+) at 37°C. After incubation for 30 minutes, cell samples were immediately analyzed by flow cytometry to determine the average fluorescence per cell at time zero (0 hours). Retained cell samples were subsequently washed twice by centrifugation, resuspended in DMEM+, maintained at 37°C for 2 hours and then analyzed by flow cytometry. The decrease in the average fluorescence intensity per cell in these samples relative to the time zero samples indicates the extent of intracellular dye leakage during the 2-hour incubation period.
BCECF AM
BCECF AM (B1150, B1170, B3051) is extensively used for detecting cytotoxicity and for determining the ability of surviving cells to proliferate.![]() The intracellular hydrolysis product of BCECF AM, BCECF (B1151, Probes Useful at Near-Neutral pH—Section 20.2), has 4–5 negative charges, a property that considerably improves its cell retention in viable cells over that of fluorescein or carboxyfluorescein (Figure 15.2.1). However, because the emission intensity of BCECF is only half-maximal at pH 7.0 (pKa = 6.98)—and is even further reduced in a cell's acidic compartments—the signal intensity of BCECF may be less than optimal in some cell viability and cell adhesion assays.
The intracellular hydrolysis product of BCECF AM, BCECF (B1151, Probes Useful at Near-Neutral pH—Section 20.2), has 4–5 negative charges, a property that considerably improves its cell retention in viable cells over that of fluorescein or carboxyfluorescein (Figure 15.2.1). However, because the emission intensity of BCECF is only half-maximal at pH 7.0 (pKa = 6.98)—and is even further reduced in a cell's acidic compartments—the signal intensity of BCECF may be less than optimal in some cell viability and cell adhesion assays.
Using monoclonal antibodies known to either enhance or inhibit natural killer (NK) cell function, researchers found that BCECF AM was at least as effective as 51Cr for measuring NK activity. Furthermore, the fluorescence-based assay could be performed with smaller samples.![]()
Fluorescein Diacetate
Fluorescein diacetate (FDA, F1303) was one of the first probes to be used as a fluorescent indicator of cell viability. FDA is still occasionally used to detect cell adhesion or, in combination with propidium iodide (P1304MP, P3566, P21493), to determine cell viability. However, fluorescein (F1300, Probes Useful at Near-Neutral pH—Section 20.2), which is formed by intracellular hydrolysis of FDA, rapidly leaks from cells (Figure 15.2.1). Thus, other cell-permeant dyes such calcein AM and BCECF AM are now preferred for cell viability assays.
Carboxyfluorescein Diacetate and Its Derivatives
The high leakage rate of fluorescein from cells prompted the development of carboxy- fluorescein diacetate (CFDA), which was originally used to measure intracellular pH but was soon adapted for use as a cell viability indicator. Upon hydrolysis by intracellular nonspecific esterases, CFDA forms carboxyfluorescein (C194, C1904; Polar Tracers—Section 14.3). As compared with fluorescein, carboxyfluorescein contains extra negative charges and is therefore better retained in cells 6 (Figure 15.2.1). CFDA is moderately permeant to most cell membranes, with uptake greater at pH 6.2 than at pH 7.4. The mixed-isomer preparation of CFDA (5(6)-CFDA, C195) is usually adequate for cell viability measurements; however, we also prepare high-purity single isomers of CFDA (C1361, C1362). In addition, we offer the electrically neutral AM ester of CFDA (5-CFDA, AM; C1354), which can be loaded into cells at lower concentrations than CFDA. Upon hydrolysis by intracellular esterases, this AM ester also yields carboxyfluorescein. CFDA, CFDA AM and sulfoforescein diacetate (see below) have been proposed for detection of living organisms on Mars. Hemoglobin can be used to quench extracellular fluorescence due to leakage of probes or leakage of products, such as fluorescein or carboxyfluorescein. Alternatively, antibodies directed against the fluorescein hapten (Anti-Dye and Anti-Hapten Antibodies—Section 7.4, Anti-fluorophore and anti-hapten antibodies—Table 7.8) or the membrane-impermeant reagent trypan blue can be used to quench low levels of extracellular fluorescence of some fluorescein-based dyes.
CFDA has been used as a viability probe with a variety of cells, including bacteria,![]() fungi (e.g., Saccharomyces cerevisiae),
fungi (e.g., Saccharomyces cerevisiae),![]() spermatozoa,
spermatozoa,![]() natural killer (NK) cells
natural killer (NK) cells ![]() and tumor cells.
and tumor cells.![]() Cytotoxicity assays using either CFDA or 5-(and 6-)carboxy-2',7'-dichlorofluorescein diacetate (carboxy-DCFDA, C369) show good correlation with results obtained using the radioisotopic 51Cr-release method.
Cytotoxicity assays using either CFDA or 5-(and 6-)carboxy-2',7'-dichlorofluorescein diacetate (carboxy-DCFDA, C369) show good correlation with results obtained using the radioisotopic 51Cr-release method.![]() With its low pKa, carboxy-DCFDA is frequently used as a selective probe for the relatively acidic yeast vacuole.
With its low pKa, carboxy-DCFDA is frequently used as a selective probe for the relatively acidic yeast vacuole.![]() Oregon Green 488 carboxylic acid diacetate (carboxy-DFFDA, O6151) also exhibits a low pKa (~4.7) and may be similarly useful as a vital stain. Its intracellular hydrolysis product—Oregon Green 488 carboxylic acid (O6146, Fluorescein, Oregon Green and Rhodamine Green Dyes—Section 1.5)—is more photostable than carboxyfluorescein.
Oregon Green 488 carboxylic acid diacetate (carboxy-DFFDA, O6151) also exhibits a low pKa (~4.7) and may be similarly useful as a vital stain. Its intracellular hydrolysis product—Oregon Green 488 carboxylic acid (O6146, Fluorescein, Oregon Green and Rhodamine Green Dyes—Section 1.5)—is more photostable than carboxyfluorescein.
Sulfofluorescein Diacetate
Sulfofluorescein diacetate (SFDA, S1129), which is converted by intracellular esterases to fluorescein sulfonic acid (F1130, Polar Tracers—Section 14.3), is more polar than CFDA and consequently may be more difficult to load into some viable cells. However, SFDA's polar hydrolysis product, fluorescein sulfonic acid, is better retained in viable cells than is carboxyfluorescein.![]() SFDA was used to stain live bacteria and fungi in soil; little interference from autofluorescence of soil minerals or detritus was observed.
SFDA was used to stain live bacteria and fungi in soil; little interference from autofluorescence of soil minerals or detritus was observed.![]()
CellTracker Green CMFDA
The CellTracker dyes are thiol-reactive fluorescent dyes that are retained in many live cells through several generations (Figure 15.2.1) and are not transferred to adjacent cells in a population (![]() ,
, ![]() ,
, ![]() ), except possibly through gap junctions. These dyes represent a significant breakthrough in the cellular retention of fluorescent probes and are ideal long-term tracers for transplanted cells or tissues (Membrane-Permeant Reactive Tracers—Section 14.2).
), except possibly through gap junctions. These dyes represent a significant breakthrough in the cellular retention of fluorescent probes and are ideal long-term tracers for transplanted cells or tissues (Membrane-Permeant Reactive Tracers—Section 14.2).
CellTracker Green CMFDA (C2925, C7025) freely diffuses into the cell, where its weakly thiol-reactive chloromethyl moieties react with intracellular thiols and their acetate groups are cleaved by cytoplasmic esterases (Figure 15.2.2), generating the fluorescent product (![]() ). The other CellTracker probes (coumarin, BODIPY and tetramethylrhodamine derivatives; Membrane-Permeant Reactive Tracers—Section 14.2) do not require enzymatic cleavage to activate their fluorescence. Because the CellTracker dyes may react with both glutathione and proteins, cells with membranes that become compromised after staining may retain some residual fluorescent conjugates. However, use of a membrane-impermeant probe such as propidium iodide (P1304MP, P3566, P21493), SYTOX Blue (S11348, S34857), SYTOX Orange (S11368), SYTOX Red (S34859), SYTOX AADvanced (S10274, S10349) or one of our "dimeric" or "monomeric" nucleic acid stains (see below) in combination with CellTracker Green CMFDA should permit relatively long-term cytotoxicity assays. CellTracker Green CMFDA and ethidium homodimer-1 (EthD-1, E1169) have been used to detect viable and nonviable cells in rat and human coronary and internal thoracic arteries sampled at autopsy
). The other CellTracker probes (coumarin, BODIPY and tetramethylrhodamine derivatives; Membrane-Permeant Reactive Tracers—Section 14.2) do not require enzymatic cleavage to activate their fluorescence. Because the CellTracker dyes may react with both glutathione and proteins, cells with membranes that become compromised after staining may retain some residual fluorescent conjugates. However, use of a membrane-impermeant probe such as propidium iodide (P1304MP, P3566, P21493), SYTOX Blue (S11348, S34857), SYTOX Orange (S11368), SYTOX Red (S34859), SYTOX AADvanced (S10274, S10349) or one of our "dimeric" or "monomeric" nucleic acid stains (see below) in combination with CellTracker Green CMFDA should permit relatively long-term cytotoxicity assays. CellTracker Green CMFDA and ethidium homodimer-1 (EthD-1, E1169) have been used to detect viable and nonviable cells in rat and human coronary and internal thoracic arteries sampled at autopsy ![]() and in connective tissue explants.
and in connective tissue explants.![]()

Chloromethyl SNARF-1 Acetate
Chloromethyl SNARF-1 acetate (C6826) is the only cell-tracking dye (and pH indicator) that exhibits bright red cytoplasmic fluorescence (![]() ) when excited at the same wavelengths used to excite the green-fluorescent hydrolysis product of CMFDA. The spectral characteristics of these two dyes permit simultaneous tracking of two cell populations by either fluorescence microscopy or flow cytometry. The large Stokes shift of the SNARF fluorophore also makes chloromethyl SNARF-1 acetate useful as a viability indicator in cells that exhibit green autofluorescence when excited by the 488 nm spectral line of the argon-ion laser.
) when excited at the same wavelengths used to excite the green-fluorescent hydrolysis product of CMFDA. The spectral characteristics of these two dyes permit simultaneous tracking of two cell populations by either fluorescence microscopy or flow cytometry. The large Stokes shift of the SNARF fluorophore also makes chloromethyl SNARF-1 acetate useful as a viability indicator in cells that exhibit green autofluorescence when excited by the 488 nm spectral line of the argon-ion laser.
Carboxynaphthofluorescein Diacetate
Carboxynaphthofluorescein diacetate (C13196), which is cleaved by intracellular esterases to yield red-fluorescent carboxynaphthofluorescein (excitation/emission maxima ~598/668 nm), is the only long-wavelength tracer of this type that can be passively loaded into live cells.![]() Like chloromethyl SNARF-1 acetate, carboxynaphthofluorescein diacetate is usually used in combination with a green-fluorescent tracer for detecting cell–cell interactions.
Like chloromethyl SNARF-1 acetate, carboxynaphthofluorescein diacetate is usually used in combination with a green-fluorescent tracer for detecting cell–cell interactions.
FilmTracer Biofilm Stains
Bacterial biofilms present a unique set of challenges for fluorescent staining and subsequent imaging. A typical biofilm not only exhibits heterogeneous thickness throughout the surface, placing stringent restrictions on stain penetration, but also contains regions of widely varying environmental conditions. Evidence suggests that bacterial cells exist in various physiological states within these biofilm microenvironments. Furthermore, biofilms contain many poorly defined components (e.g., the extracellular polymeric matrix) that differ with species and conditions.
FilmTracer calcein violet, FilmTracer calcein green and FilmTracer calcein red-orange biofilm stains (F10320, F10322, F10319) are acetoxymethyl (AM) ester derivatives of fluorescent indicators that provide reliable indication of esterase activity in live cells and are of particular use in biofilm applications. FilmTracer calcein violet and FilmTracer calcein green biofilm stains are colorless and nonfluorescent until the AM ester is hydrolyzed. FilmTracer calcein red-orange biofilm stain is fluorescent prior to cleavage; however, the intracellular fluorescence is much brighter than background fluorescence after rinsing.
FM dyes are lipophilic styryl compounds useful as general-purpose probes for investigating endocytosis and for identifying cell membrane boundaries (Tracers for Membrane Labeling—Section 14.4). These styryl dyes are easily applied to cells, where they bind rapidly and reversibly to the plasma membrane with strong fluorescence enhancement. ![]() FilmTracer FM 1-43 green biofilm cell stain (F10317) has been used successfully to stain the cell bodies in a complex biofilm mileu, including Pseudomonas aeruginosa (Figure 15.2.3), Escherichia coli, Staphylococcus sp., Acidothiobacillus caldus and Vibrio cholerae.
FilmTracer FM 1-43 green biofilm cell stain (F10317) has been used successfully to stain the cell bodies in a complex biofilm mileu, including Pseudomonas aeruginosa (Figure 15.2.3), Escherichia coli, Staphylococcus sp., Acidothiobacillus caldus and Vibrio cholerae.![]()
Our SYPRO Ruby stain labels most classes of proteins, including glycoproteins, phosphoproteins, lipoproteins, calcium binding proteins, fibrillar proteins and other proteins that are difficult to stain ![]() (Protein Detection on Gels, Blots and Arrays—Section 9.3). FilmTracer SYPRO Ruby Biofilm Matrix Stain (F10318) is a specially formulated version of the SYPRO Ruby stain and has been found to stain the matrix of Pseudomonas aeruginosa (ATCC 15442) and some strains of Escherichia coli; it does not stain E. coli K-12, which does not produce cellulose. As with all the biofilm stains, staining patterns may vary depending upon the organism and the matrix composition.
(Protein Detection on Gels, Blots and Arrays—Section 9.3). FilmTracer SYPRO Ruby Biofilm Matrix Stain (F10318) is a specially formulated version of the SYPRO Ruby stain and has been found to stain the matrix of Pseudomonas aeruginosa (ATCC 15442) and some strains of Escherichia coli; it does not stain E. coli K-12, which does not produce cellulose. As with all the biofilm stains, staining patterns may vary depending upon the organism and the matrix composition.
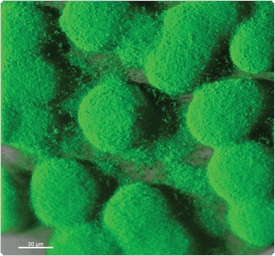
Figure 15.2.3 FilmTracer FM 1-43 green biofilm cell stain (F10317) applied to aPseudomonas aeruginosa biofilm. FilmTracer FM 1-43 green biofilm cell stain appears to bind to the cell membrane. This stain has been shown to work equally well on Staphylococcus epidermidis, Pseudomonas aeruginosa and Escherichia coli, exhibiting exceptional cell specificity in each case. The image was obtained using a Leica TCS-SP2 AOBS confocal microscope and a 63×/0.9 NA water immersion objective. Image contributed by Betsey Pitts and Ellen Swogger, Center for Biofilm Engineering, Montana State University.
Viability assessments of animal cells, bacteria and yeast frequently employ polar and therefore cell-impermeant nucleic acid stains to detect the dead-cell population. Nucleic acid stains are most often used in combination with intracellular esterase substrates (see above), membrane-permeant nucleic acid stains (see below), membrane potential–sensitive probes (Probes for Membrane Potential—Chapter 22), organelle probes (Probes for Organelles—Chapter 12) or cell-permeant indicators (Indicators for Ca2+, Mg2+, Zn2+ and Other Metal Ions—Chapter 19, pH Indicators—Chapter 20 and Indicators for Na+, K+, Cl– and Miscellaneous Ions—Chapter 21) to simultaneously detect the live-cell population. Although many other cell-impermeant dyes can be used to detect dead cells, the high concentrations of nucleic acids in cells, coupled with the large fluorescence enhancement exhibited by most of our nucleic acid stains upon binding, make cell-impermeant nucleic acid stains the logical candidates for viability probes. See Cell membrane–impermeant cyanine nucleic acid stains—Table 8.2 for a list of several cell-impermeant nucleic acid stains and Nucleic Acid Stains—Section 8.1 for a general discussion of dye binding to nucleic acids.
SYTOX Nucleic Acid Stains
Many polar nucleic acid stains will enter eukaryotic cells with damaged plasma membranes yet will not stain dead bacteria with damaged plasma membranes. SYTOX Green nucleic acid stain (S7020) is a high-affinity probe that easily penetrates eukaryotic cells and both gram-positive and gram-negative bacteria with compromised plasma membranes, yet is completely excluded from live cells.![]() After brief incubation with the SYTOX Green nucleic acid stain, dead bacteria fluoresce bright green when excited with the 488 nm spectral line of the argon-ion laser or any other 470–490 nm source. These properties, combined with its ~1000-fold fluorescence enhancement upon nucleic acid binding, make our SYTOX Green stain a simple and quantitative dead-cell indicator for use with fluorescence microscopes, fluorometers, fluorescence microplate readers or flow cytometers (Figure 15.2.4). We have taken advantage of the sensitivity of the SYTOX Green nucleic acid stain in our ViaGram Red+ Bacterial Gram Stain and Viability Kit (V7023) and in our Single Channel Annexin V/Dead Cell Apoptosis Kit (V13240, Assays for Apoptosis—Section 15.5). A significant application of the SYTOX Green nucleic acid stain is the high-throughput screening of bacteria for antibiotic susceptibility by fluorescence microscopy, by flow cytometry or in a fluorescence microplate reader.
After brief incubation with the SYTOX Green nucleic acid stain, dead bacteria fluoresce bright green when excited with the 488 nm spectral line of the argon-ion laser or any other 470–490 nm source. These properties, combined with its ~1000-fold fluorescence enhancement upon nucleic acid binding, make our SYTOX Green stain a simple and quantitative dead-cell indicator for use with fluorescence microscopes, fluorometers, fluorescence microplate readers or flow cytometers (Figure 15.2.4). We have taken advantage of the sensitivity of the SYTOX Green nucleic acid stain in our ViaGram Red+ Bacterial Gram Stain and Viability Kit (V7023) and in our Single Channel Annexin V/Dead Cell Apoptosis Kit (V13240, Assays for Apoptosis—Section 15.5). A significant application of the SYTOX Green nucleic acid stain is the high-throughput screening of bacteria for antibiotic susceptibility by fluorescence microscopy, by flow cytometry or in a fluorescence microplate reader.![]()
The SYTOX Green nucleic acid stain as a tool for viability assessment is not restricted to bacteria; it is also a very effective cell-impermeant counterstain in eukaryotic systems (Probes for the Nucleus—Section 12.5). It can be used in conjunction with blue- and red-fluorescent labels for multiparameter analyses in fixed cells and tissue sections (![]() ,
, ![]() ,
, ![]() ). Furthermore, it should be possible to combine the SYTOX Green nucleic acid stain with one of the membrane-permeant nucleic acid stains in our SYTO Red-, SYTO Blue- or SYTO Orange-Fluorescent Nucleic Acid Stain Sampler Kits (S11340, S11350, S11360) for two-color visualization of dead and live cells.
). Furthermore, it should be possible to combine the SYTOX Green nucleic acid stain with one of the membrane-permeant nucleic acid stains in our SYTO Red-, SYTO Blue- or SYTO Orange-Fluorescent Nucleic Acid Stain Sampler Kits (S11340, S11350, S11360) for two-color visualization of dead and live cells.
Like the SYTOX Green reagent, our SYTOX Orange (S11368) and SYTOX Blue nucleic acid stains (5 mM solution in dimethylsulfoxide (DMSO), S11348); 1 mM solution in DMSO, S34857) are high-affinity nucleic acid stains that only penetrate cells with compromised plasma membranes. The SYTOX Orange nucleic acid stain (S11368, ![]() ) has absorption/emission maxima of 547/570 nm when bound to DNA and is optimally detected using filters appropriate for rhodamine dyes. As with the other SYTOX dyes, the SYTOX Orange stain is virtually nonfluorescent except when bound to nucleic acids and can be used to detect cells that have compromised membranes without a wash step
) has absorption/emission maxima of 547/570 nm when bound to DNA and is optimally detected using filters appropriate for rhodamine dyes. As with the other SYTOX dyes, the SYTOX Orange stain is virtually nonfluorescent except when bound to nucleic acids and can be used to detect cells that have compromised membranes without a wash step
Our SYTOX Blue nucleic acid stain labels both DNA and RNA with extremely bright fluorescence centered near 480 nm, making it an excellent fluorescent indicator of cell viability (![]() ). Unlike many blue-fluorescent dyes, the SYTOX Blue stain is efficiently excited by the 405 nm violet diode laser. The brightness of the SYTOX Blue complex with nucleic acids allows sensitive detection of stained cells with fluorometers, fluorescence microplate readers, flow cytometers and epifluorescence microscopes. Quantitation of membrane-compromised bacterial cells carried out with the SYTOX Blue stain yields results identical to those obtained in parallel assays using the SYTOX Green stain. Like the SYTOX Green stain, the SYTOX Blue stain does not interfere with bacterial cell growth. Because their emission spectra overlap somewhat, we have found that it is not ideal to use SYTOX Blue stain and green-fluorescent dyes together in the same application, except when the green-fluorescent dye is excited beyond the absorption of the SYTOX Blue dye (e.g., at >480 nm). However, emission of the SYTOX Blue complex with nucleic acids permits clear discrimination from red- and orange-fluorescent probes, facilitating development of multicolor assays with minimal spectral overlap between signals.
). Unlike many blue-fluorescent dyes, the SYTOX Blue stain is efficiently excited by the 405 nm violet diode laser. The brightness of the SYTOX Blue complex with nucleic acids allows sensitive detection of stained cells with fluorometers, fluorescence microplate readers, flow cytometers and epifluorescence microscopes. Quantitation of membrane-compromised bacterial cells carried out with the SYTOX Blue stain yields results identical to those obtained in parallel assays using the SYTOX Green stain. Like the SYTOX Green stain, the SYTOX Blue stain does not interfere with bacterial cell growth. Because their emission spectra overlap somewhat, we have found that it is not ideal to use SYTOX Blue stain and green-fluorescent dyes together in the same application, except when the green-fluorescent dye is excited beyond the absorption of the SYTOX Blue dye (e.g., at >480 nm). However, emission of the SYTOX Blue complex with nucleic acids permits clear discrimination from red- and orange-fluorescent probes, facilitating development of multicolor assays with minimal spectral overlap between signals.
SYTOX Red dead cell stain (S34859) is a simple and quantitative single-step dead-cell indicator for use with red laser–equipped flow cytometers. SYTOX Red dead cell stain is a high-affinity nucleic acid stain that easily penetrates cells with compromised plasma membranes but will not cross uncompromised cell membranes. After brief incubation with SYTOX Red stain, the nucleic acids of dead cells fluoresce bright red when excited with 633 or 635 nm red laser light. SYTOX Red nucleic acid stain has absorption/emission maxima of 633/660 nm when bound to DNA and is optimally detected using filters appropriate for Alexa Fluor 647 dye. SYTOX Red dead cell stain is distinct from other dead cell probes like 7-AAD and PI, which require 488 nm excitation.
Unlike the other SYTOX stains, SYTOX AADvanced Dead Cell Stain Kits (S10274, S10349) provided separate vials of dried dye and anhydrous DMSO to help ensure a stable shelf life. SYTOX AADvanced dead cell stain is a high-affinity nucleic acid stain for the detection of dead cells and analysis of cell cycle using the common 488 nm spectral line of the argon-ion laser in flow cytometry. The dye is spectrally similar to 7-AAD but exhibits rapid uptake kinetics and relatively low cellular staining variability. SYTOX AADvanced dead cell stain penetrates cells more efficiently than does 7-AAD, providing better separation of live- and dead-cell fluorescence signals. SYTOX AADvanced dead cell stain can also be used with fixed cells for DNA content analysis when paired with RNAse treatment.
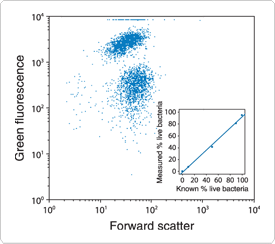
Figure 15.2.4 Quantitative flow cytometric analysis of Escherichia coli viability using the SYTOX Green nucleic acid stain (S7020). A bacterial suspension containing an equal number of live and isopropyl alcohol–killed E. coli was stained with SYTOX Green and analyzed using excitation at 488 nm. A bivariate frequency distribution for forward light scatter versus log fluorescence intensity (collected with a 510 nm longpass optical filter) shows two clearly distinct populations. When live and dead bacteria were mixed in varying proportions, a linear relationship between the population numbers and the actual percentage of live cells in the sample was obtained (see inset).
Dimeric and Monomeric Cyanine Dyes
The dimeric and monomeric cyanine dyes in the TOTO and TO-PRO series (Cell membrane–impermeant cyanine nucleic acid stains—Table 8.2) are essentially nonfluorescent unless bound to nucleic acids and have extinction coefficients 10–20 times greater than that of DNA-bound propidium iodide. Spectra of the nucleic acid–bound dyes cover the entire visible spectrum and into the infrared region (Figure 15.2.5). These dyes are typically impermeant to the membranes of live cells ![]() but brightly stain dead cells that have compromised membranes. However, YO-PRO-1 and other polar stains are taken up by some live cells via the P2X1–7receptors
but brightly stain dead cells that have compromised membranes. However, YO-PRO-1 and other polar stains are taken up by some live cells via the P2X1–7receptors ![]() as well as by apoptotic cells (Assays for Apoptosis—Section 15.5), so care needs to be taken when assaying cell viability with nucleic acid stains. The POPO-1 and BOBO-1 dyes may be useful blue-fluorescent dead-cell stains, and the YOYO-3 and TOTO-3 dyes and the corresponding YO-PRO-3 and TO-PRO-3 dyes have excitation maxima beyond 600 nm when bound to DNA. Our JOJO-1 and JO-PRO-1 dyes exhibit orange fluorescence (~545 nm) upon binding to nucleic acids and can be excited with a 532 nm Nd:YAG laser. The LOLO-1 nucleic acid stain has longer-wavelength fluorescence (~580 nm). Our Nucleic Acid Stains Dimer Sampler Kit (N7565) provides a total of eight cyanine dyes that span the visible spectrum. This kit should be useful when screening dyes for their utility in viability and cytotoxicity assays.
as well as by apoptotic cells (Assays for Apoptosis—Section 15.5), so care needs to be taken when assaying cell viability with nucleic acid stains. The POPO-1 and BOBO-1 dyes may be useful blue-fluorescent dead-cell stains, and the YOYO-3 and TOTO-3 dyes and the corresponding YO-PRO-3 and TO-PRO-3 dyes have excitation maxima beyond 600 nm when bound to DNA. Our JOJO-1 and JO-PRO-1 dyes exhibit orange fluorescence (~545 nm) upon binding to nucleic acids and can be excited with a 532 nm Nd:YAG laser. The LOLO-1 nucleic acid stain has longer-wavelength fluorescence (~580 nm). Our Nucleic Acid Stains Dimer Sampler Kit (N7565) provides a total of eight cyanine dyes that span the visible spectrum. This kit should be useful when screening dyes for their utility in viability and cytotoxicity assays.
One cell viability assay utilizes YOYO-1 fluorescence before and after treatment with digitonin as a measure of the dead cells and total cells, respectively, in the sample.![]() The YOYO-3 dye was used as a stain for dead cells in an assay designed to correlate cell cycle with metabolism in single cells.
The YOYO-3 dye was used as a stain for dead cells in an assay designed to correlate cell cycle with metabolism in single cells.![]() In addition to their use as dead-cell stains, both the dimeric and monomeric cyanine dyes are also proving useful for staining viruses. TOTO-1 dye–staining and flow cytometric analysis gave better discrimination of live and dead lactic acid bacteria in several species than did propidium iodide.
In addition to their use as dead-cell stains, both the dimeric and monomeric cyanine dyes are also proving useful for staining viruses. TOTO-1 dye–staining and flow cytometric analysis gave better discrimination of live and dead lactic acid bacteria in several species than did propidium iodide.![]() Viruses stained with the YOYO-1 and POPO-1 dyes have been employed to identify and quantitate bacteria and cyanobacteria in marine microbial communities.
Viruses stained with the YOYO-1 and POPO-1 dyes have been employed to identify and quantitate bacteria and cyanobacteria in marine microbial communities.![]() The YO-PRO-1 dye has been used to count viruses in marine and freshwater environments by epifluorescence microscopy
The YO-PRO-1 dye has been used to count viruses in marine and freshwater environments by epifluorescence microscopy ![]() and is also selectively permeant to apoptotic cells
and is also selectively permeant to apoptotic cells ![]() (Assays for Apoptosis—Section 15.5). TO-PRO-3 (T3605, Assays for Cell Enumeration, Cell Proliferation and Cell Cycle—Section 15.4) has been utilized to demonstrate transient permeabilization of bacterial by sublethal doses of antibiotics.
(Assays for Apoptosis—Section 15.5). TO-PRO-3 (T3605, Assays for Cell Enumeration, Cell Proliferation and Cell Cycle—Section 15.4) has been utilized to demonstrate transient permeabilization of bacterial by sublethal doses of antibiotics.![]()

Figure 15.2.5 Normalized fluorescence emission spectra of DNA-bound cyanine dimers, identified by the color key on the sidebar.
Ethidium and Propidium Dyes
The red-fluorescent, cell-impermeant ethidium and propidium dyes—ethidium bromide, ethidium homodimer-1 (EthD-1, E1169) and propidium iodide (P1304MP, P3566, P21493)—can all be excited by the argon-ion laser and are therefore useful for detecting and sorting dead cells by flow cytometry.![]() Moreover, these dyes have large Stokes shifts and may be used in combination with fluorescein-based probes (such as calcein, CellTracker Green CMFDA or BCECF) or green-fluorescent SYTO dyes (Cell-permeant cyanine nucleic acid stains—Table 8.3) for two-color applications (Figure 15.2.6). Both propidium iodide and ethidium bromide have been extensively used to detect dead or dying cells,
Moreover, these dyes have large Stokes shifts and may be used in combination with fluorescein-based probes (such as calcein, CellTracker Green CMFDA or BCECF) or green-fluorescent SYTO dyes (Cell-permeant cyanine nucleic acid stains—Table 8.3) for two-color applications (Figure 15.2.6). Both propidium iodide and ethidium bromide have been extensively used to detect dead or dying cells,![]() although ethidium bromide may be somewhat less reliable because it is not as highly charged. EthD-1 and propidium iodide are superior to ethidium bromide for two-color flow cytometric viability assays in which either BCECF AM or calcein AM is used as the live-cell stain because their spectra do not overlap as much with those of the green-fluorescent esterase probes.
although ethidium bromide may be somewhat less reliable because it is not as highly charged. EthD-1 and propidium iodide are superior to ethidium bromide for two-color flow cytometric viability assays in which either BCECF AM or calcein AM is used as the live-cell stain because their spectra do not overlap as much with those of the green-fluorescent esterase probes.![]()
With its high affinity for DNA and low membrane permeability,![]() EthD-1 is often the preferred red-fluorescent dead-cell indicator. EthD-1 binds to nucleic acids 1000 times more tightly than does ethidium bromide and undergoes about a 40-fold enhancement of fluorescence upon binding.
EthD-1 is often the preferred red-fluorescent dead-cell indicator. EthD-1 binds to nucleic acids 1000 times more tightly than does ethidium bromide and undergoes about a 40-fold enhancement of fluorescence upon binding.![]() When used as a viability indicator, EthD-1 typically does not require a wash step. Also, the high affinity of EthD-1 permits the use of very low concentrations to stain dead cells, thus avoiding the use of large quantities of the potentially hazardous ethidium bromide or propidium iodide. EthD-1, the dead-cell indicator in our LIVE/DEAD Viability/Cytotoxicity Kit (L3224,, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3), has been used alone
When used as a viability indicator, EthD-1 typically does not require a wash step. Also, the high affinity of EthD-1 permits the use of very low concentrations to stain dead cells, thus avoiding the use of large quantities of the potentially hazardous ethidium bromide or propidium iodide. EthD-1, the dead-cell indicator in our LIVE/DEAD Viability/Cytotoxicity Kit (L3224,, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3), has been used alone ![]() or in combination with calcein AM
or in combination with calcein AM ![]() to detect tumor necrosis factor activity and to assay neuronal cell death.
to detect tumor necrosis factor activity and to assay neuronal cell death.![]() Ethidium homodimer-2 (E3599), which under our trademark DEAD Red is used as the necrotic-cell indicator in our LIVE/DEAD Reduced Biohazard Cell Viability Kit #1 (L7013, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3), has a particularly low dissociation rate from cellular nucleic acids, permitting its use for selective marking of dead-cell populations that need to be observed over several hours. Our DEAD Red nucleic acid stain has proven useful for determining brain stem lesion size in vivo in rats following a neurotoxin injection.
Ethidium homodimer-2 (E3599), which under our trademark DEAD Red is used as the necrotic-cell indicator in our LIVE/DEAD Reduced Biohazard Cell Viability Kit #1 (L7013, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3), has a particularly low dissociation rate from cellular nucleic acids, permitting its use for selective marking of dead-cell populations that need to be observed over several hours. Our DEAD Red nucleic acid stain has proven useful for determining brain stem lesion size in vivo in rats following a neurotoxin injection.![]() Live and dead cells of the yeast-like fungus Aureobasidium pullulans have been identified on microscope slides as well as leaf surfaces using CellTracker Blue CMAC (C2110, Membrane-Permeant Reactive Tracers—Section 14.2) in conjunction with the DEAD Red nucleic acid stain.
Live and dead cells of the yeast-like fungus Aureobasidium pullulans have been identified on microscope slides as well as leaf surfaces using CellTracker Blue CMAC (C2110, Membrane-Permeant Reactive Tracers—Section 14.2) in conjunction with the DEAD Red nucleic acid stain.![]()
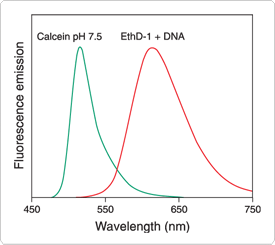
Figure 15.2.6 Normalized fluorescence emission spectra of calcein (C481) and DNA-bound ethidium homodimer-1 (EthD-1, E1169), illustrating the clear spectral separation that allows simultaneous visualization of live and dead eukaryotic cells with Molecular Probes LIVE/DEAD Viability/Cytotoxicity Kit (L3224).
Ethidium Monoazide
Ethidium monoazide (E1374) is a fluorescent photoaffinity label that, after photolysis, binds covalently to nucleic acids in solution and in cells with compromised membranes.![]() A mixed population of live and dead cells incubated with this membrane-impermeant dye can be illuminated with a visible-light source, washed, fixed and then analyzed in order to determine the viability of the cells at the time of photolysis. Thus, ethidium monoazide reduces some of the hazards inherent in working with pathogenic samples because, once stained, samples can be treated with fixatives before analysis by fluorescence microscopy or flow cytometry.
A mixed population of live and dead cells incubated with this membrane-impermeant dye can be illuminated with a visible-light source, washed, fixed and then analyzed in order to determine the viability of the cells at the time of photolysis. Thus, ethidium monoazide reduces some of the hazards inherent in working with pathogenic samples because, once stained, samples can be treated with fixatives before analysis by fluorescence microscopy or flow cytometry.![]() Immunocytochemical analyses requiring fixation are also compatible with this ethidium monoazide–based viability assay. We have developed an alternative two-color fluorescence-based assay for determining the original viability of fixed samples that employs our cell-permeant, green-fluorescent SYTO 10 and cell-impermeant, red-fluorescent DEAD Red nucleic acid stains; the LIVE/DEAD Reduced Biohazard Cell Viability Kit #1 (L7013) is described in Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3.
Immunocytochemical analyses requiring fixation are also compatible with this ethidium monoazide–based viability assay. We have developed an alternative two-color fluorescence-based assay for determining the original viability of fixed samples that employs our cell-permeant, green-fluorescent SYTO 10 and cell-impermeant, red-fluorescent DEAD Red nucleic acid stains; the LIVE/DEAD Reduced Biohazard Cell Viability Kit #1 (L7013) is described in Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3.
Hexidium Iodide: A Fluorescent Gram Stain
Ethidium bromide is only marginally permeant to cell membranes or bacteria; however, we found that our hexidium iodide stain (H7593) has the right combination of polarity and permeability to allow it to rapidly stain most gram-positive bacteria while being excluded by the less-permeant membranes of most gram-negative bacteria.![]() Combining the red-orange–fluorescent hexidium iodide reagent with a green-fluorescent, membrane-permeant nucleic acid stain—as in our LIVE BacLight Bacterial Gram Stain Kit (L7005, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3)—enables taxonomic classification of most bacteria in minutes, using a single staining solution, no fixatives and no wash steps. This rapid gram stain assay should be useful in both clinical and research settings. The validity of using hexidium iodide in combination with the SYTO 13 green-fluorescent nucleic acid stain to correctly predict the gram sign of 45 clinically relevant organisms, including several known to be gram variable, has been demonstrated.
Combining the red-orange–fluorescent hexidium iodide reagent with a green-fluorescent, membrane-permeant nucleic acid stain—as in our LIVE BacLight Bacterial Gram Stain Kit (L7005, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3)—enables taxonomic classification of most bacteria in minutes, using a single staining solution, no fixatives and no wash steps. This rapid gram stain assay should be useful in both clinical and research settings. The validity of using hexidium iodide in combination with the SYTO 13 green-fluorescent nucleic acid stain to correctly predict the gram sign of 45 clinically relevant organisms, including several known to be gram variable, has been demonstrated.![]() The method of use of hexidium iodide as a gram stain is described further in Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3.
The method of use of hexidium iodide as a gram stain is described further in Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3.
SYTO Nucleic Acid Stains
Our SYTO family of dyes, all of which are listed in Cell-permeant cyanine nucleic acid stains—Table 8.3, are essentially nonfluorescent until they bind to nucleic acids, whereupon their fluorescence quantum yield may increase by 1000-fold or more. These dyes are freely permeant to most cells, although their rate of uptake and ultimate staining pattern may be cell dependent. Their affinity for nucleic acids is moderate and they can be displaced by higher-affinity nucleic acid stains such as SYTOX Green, propidium iodide, the ethidium dimers and all of the monomeric and dimeric nucleic acid stains described above. Because the membrane of intact cells offers a barrier to entry of these higher-affinity nucleic acid stains, it is common to combine, for instance, a green-fluorescent SYTO dye with a red-fluorescent, high-affinity nucleic acid stain such as propidium iodide, one of the ethidium homodimers, or TOTO-3 for simultaneous staining of the live- and dead-cell populations. Although the green-fluorescent SYTO dye will still bind to nucleic acids in dead cells, it will be displaced or its fluorescence quenched by the red-fluorescent dye, resulting in a yellow-, orange- or red-fluorescent dead-cell population. This principle is the basis of our LIVE/DEAD BacLight Bacterial Viability Kits (L7007, L7012, L13152), our LIVE/DEAD Sperm Viability Kit (L7011) and our LIVE BacLight Bacterial Gram Stain Kit (L7005), which are all discussed in Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3. Four sampler kits of the SYTO dyes (S7572, S11340, S11350, S11360) provide a total of 27 SYTO dyes with emission maxima that range from 441 nm to 678 nm. All of the SYTO dyes in the sampler kits are also available individually (Nucleic Acid Stains—Section 8.1), as well as several other SYTO green-fluorescent (S32704, S34854, S34855) nucleic acid stains. The SYTO 13 green-fluorescent nucleic acid stain (S7575) has been used in combination with:
- Ethidium bromide for studies of tissue cryopreservation

- Hexidium iodide for simultaneous viability and gram sign of clinically relevant bacteria

- Ethidium homodimer-1 for quantitation of neurotoxicity

- Propidium iodide to detect the effects of surfactants on Escherichia coli viability

SYTO RNASelect Green-Fluorescent Cell Stain
SYTO RNASelect green-fluorescent cell stain (S32703) is a cell-permeant nucleic acid stain that selectively stains RNA (Figure 15.2.7). Although virtually nonfluorescent in the absence of nucleic acids, SYTO RNASelect stain exhibits bright green fluorescence when bound to RNA (absorption/emission maxima ~490/530 nm), but only weak fluorescence when bound to DNA (Figure 15.2.8). Filter sets that are suitable for imaging cells labeled with fluorescein (FITC) will work well for imaging cells stained with SYTO RNASelect stain (![]() ,
, ![]() ).
).
Eukaryotic cells stained with the SYTO RNASelect dye show a staining pattern consistent with that of an RNA-selective probe. Maximal fluorescence is observed in the nucleoli, with faint fluorescence throughout the nucleus. Weak fluorescence is also seen throughout the cytoplasm, predominantly associated with mitochondria. The RNA localization of the SYTO RNASelect stain is further supported by RNase and DNase treatments: 1) upon treatment with RNase, the nucleolar and cytoplasmic intensities are significantly reduced, as compared with control cells; 2) upon treatment with DNase, there is no significant loss of fluorescence; and 3) upon treatment with both RNase and DNase, the staining pattern is the same as that observed with RNase treatment alone.
Because the SYTO RNASelect green-fluorescent cell stain is cell permeant, it is suitable for use in live cells. After the cells have been stained, they may be fixed in methanol with minimal loss of the staining pattern. If desired, cells can also be fixed in methanol before staining RNA with the SYTO RNASelect stain. Fixation with formaldehyde alters the staining pattern and is not recommended.
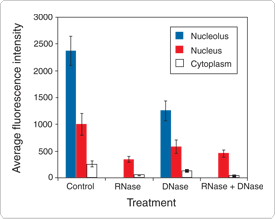
Figure 15.2.7 Methanol-fixed bovine pulmonary artery endothelial cells treated with RNase, DNase or both, and then labeled with SYTO RNASelect Green cell stain (S32703). Removal of RNA with RNase prevented nucleolar labeling and greatly decreased nuclear and cytoplasmic labeling. Use of DNase resulted in less of a loss of label intensity in these cell compartments, reflecting the RNA-selective nature of this dye.

Figure 15.2.8 Absorption (A) and fluorescence emission (B) spectra of SYTO RNASelect green-fluorescent cell stain (S32703) in the presence of Escherichia coli DNA or in buffer alone.
FUN 1 Dye: A Unique Stain for Assessing Viability of Yeast and Fungi
While structurally related to the SYTO dyes, FUN 1 dye (F7030) contains substituents that apparently make them chemically reactive with intracellular components of yeast, provided that the yeast are metabolically active. FUN 1 dye is freely taken up by several species of yeast and fungi and converted from a diffusely distributed pool of yellow-green–fluorescent intracellular stain into compact red-orange– or yellow-orange–fluorescent intravacuolar structures, respectively (![]() ). Conversion of FUN 1 dye to products with longer-wavelength emission (Figure 15.2.9) requires both plasma membrane integrity and metabolic capability. Only metabolically active cells are marked clearly with fluorescent intravacuolar structures, while dead cells exhibit extremely bright, diffuse, yellow-green fluorescence.
). Conversion of FUN 1 dye to products with longer-wavelength emission (Figure 15.2.9) requires both plasma membrane integrity and metabolic capability. Only metabolically active cells are marked clearly with fluorescent intravacuolar structures, while dead cells exhibit extremely bright, diffuse, yellow-green fluorescence.![]() The FUN 1 cell stain is also available as a component in our LIVE/DEAD Yeast Viability Kit (L7009, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3).
The FUN 1 cell stain is also available as a component in our LIVE/DEAD Yeast Viability Kit (L7009, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3).
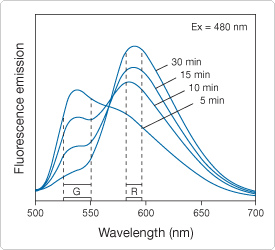
Figure 15.2.9 Fluorescence emission spectra of a Saccharomyces cerevisiaesuspension that has been stained with the FUN 1 cell stain, which is available separately (F7030) or in our LIVE/DEAD Yeast Viability Kit (L7009). After the FUN 1 reagent was added to the medium, the fluorescence emission spectrum (excited at 480 nm) was recorded in a spectrofluorometer at the indicated times during a 30-minute incubation period. The shift from green (G) to red (R) fluorescence reflects the processing of FUN 1 by metabolically active yeast cells.
7-Aminoactinomycin D
7-AAD (7-aminoactinomycin D, A1310; Assays for Cell Enumeration, Cell Proliferation and Cell Cycle—Section 15.4) is a fluorescent intercalator that undergoes a spectral shift upon association with DNA. 7-AAD/DNA complexes can be excited by the argon-ion laser and emit beyond 610 nm, making this nucleic acid stain useful for multicolor fluorescence microscopy, confocal laser-scanning microscopy and flow cytometry. 7-AAD appears to be generally excluded from live cells, although it has been reported to label the nuclear region of live cultured mouse L cells and salivary gland polytene chromosomes of Chironomus thummi thummi larvae.![]() 7-AAD is also a useful marker for apoptotic cell populations (Assays for Apoptosis—Section 15.5) and has been utilized to discriminate dead cells from apoptotic and live cells.
7-AAD is also a useful marker for apoptotic cell populations (Assays for Apoptosis—Section 15.5) and has been utilized to discriminate dead cells from apoptotic and live cells.![]() In addition, 7-AAD can be used in combination with conjugates of R-phycoerythrin (Phycobiliproteins—Section 6.4) in three-color flow cytometry protocols.
In addition, 7-AAD can be used in combination with conjugates of R-phycoerythrin (Phycobiliproteins—Section 6.4) in three-color flow cytometry protocols.
The generation of reactive oxygen species (ROS) is inevitable for aerobic organisms and, in healthy cells, occurs at a controlled rate. Under conditions of oxidative stress, ROS production is dramatically increased, resulting in subsequent alteration of membrane lipids, proteins and nucleic acids. Oxidative damage of these biomolecules is associated with aging ![]() and with a variety of pathological events, including atherosclerosis, carcinogenesis, ischemic reperfusion injury and neurodegenerative disorders.
and with a variety of pathological events, including atherosclerosis, carcinogenesis, ischemic reperfusion injury and neurodegenerative disorders.![]()
Metabolically active cells can oxidize or reduce a variety of probes, providing a measure of cell viability and overall cell health. This measure of viability is distinct from that provided by probes designed to detect esterase activity or cell permeability. Detecting oxidative activity and ROS in cells is also discussed in Generating and Detecting Reactive Oxygen Species—Section 18.2.
High-Purity Resazurin
Resazurin (R12204) has been extensively used as an oxidation–reduction indicator to detect bacteria and yeast in broth cultures and milk,![]() to assess the activity of sperm
to assess the activity of sperm ![]() and to assay bile acids
and to assay bile acids ![]() and triglycerides.
and triglycerides.![]() Resazurin reduction also occurs with other mammalian cells, including neurons,
Resazurin reduction also occurs with other mammalian cells, including neurons,![]() corneal endothelial cells,
corneal endothelial cells,![]() lymphocytes, lymphoid tumor cells and hybridoma cells.
lymphocytes, lymphoid tumor cells and hybridoma cells.![]() Furthermore, resazurin has been used in high-throughput screening assays for compounds that act against Mycobacterium tuberculosis.
Furthermore, resazurin has been used in high-throughput screening assays for compounds that act against Mycobacterium tuberculosis.![]() However, correlation of the results obtained with resazurin and bioluminescent assays for ATP has been reported to be poor.
However, correlation of the results obtained with resazurin and bioluminescent assays for ATP has been reported to be poor.![]() Resazurin has been reported to be useful for quantitatively measuring cell-mediated cytotoxicity,
Resazurin has been reported to be useful for quantitatively measuring cell-mediated cytotoxicity,![]() cell proliferation
cell proliferation ![]() and mitochondrial metabolic activity is isolated neural tissue.
and mitochondrial metabolic activity is isolated neural tissue.![]()
Dodecylresazurin: A Superior Probe for Cell Metabolic Studies
Dodecylresazurin (C12-resazurin), which is available only as a component of our Vybrant Cell Metabolic Assay Kit (V23110, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3), LIVE/DEAD Cell Vitality Assay Kit (L34951, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3) and Metabolic Activity/Annexin V/Dead Cell Apoptosis Kit (V35114, Assays for Apoptosis—Section 15.5), has several properties that make it superior to resazurin (and alamarBlue) for detecting metabolic activity in cells:
- C12-resazurin is freely permeant to the membranes of most cells.
- Less C12-resazurin is required for equivalent sensitivity relative to resazurin.
- Unlike resazurin, which yields a product (resorufin) that rapidly leaks from viable cells, the product of reduction of C12-resazurin—C12-resorufin—is relatively well retained by single cells, permitting flow cytometric assay of cell metabolism and viability on a single-cell basis.
- The fluorescence developed by reduction of C12-resazurin is directly proportional to cell number, and the assay is capable of detecting very low numbers of cells, even in a high-throughput microplate assay.
Dihydrorhodamines and Dihydrofluoresceins
Fluorescein, rhodamine and various other dyes can be chemically reduced to colorless, nonfluorescent leuco dyes. These "dihydro" derivatives are readily oxidized back to the parent dye by some reactive oxygen species (Generating and Detecting Reactive Oxygen Species—Section 18.2) and thus can serve as fluorogenic probes for detecting oxidative activity in cells and tissues.![]() Because reactive oxygen species are produced by live but not dead cells, fluorescent oxidation products that are retained in cells can be used as viability indicators for single cells or cell suspensions. Some probes that are useful for detecting oxidative activity in metabolically active cells include:
Because reactive oxygen species are produced by live but not dead cells, fluorescent oxidation products that are retained in cells can be used as viability indicators for single cells or cell suspensions. Some probes that are useful for detecting oxidative activity in metabolically active cells include:
- H2DCFDA
(2',7'-dichlorodihydrofluorescein diacetate, D399), carboxy-H2DCFDA (5-(and 6-)carboxy-2',7'-dichlorodihydrofluorescein diacetate, C400) and the acetoxymethyl ester of H2DCFDA (C2938), all of which require both intracellular deacetylation and oxidation to yield green-fluorescent products
- CM-H2DCFDA (chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester, C6827), which is analogous to H2DCFDA, except that it forms a mildly thiol-reactive fluorescent product after oxidation by metabolically active cells (Figure 15.2.10), permitting significantly longer-term measurements
- Dihydrocalcein AM (D23805), our newest dihydrofluorescein derivative, which is converted intracellularly to calcein, a green-fluorescent dye with superior cell retention (Figure 15.2.1)
- Dihydrorhodamine 123 (D632, D23806) and dihydrorhodamine 6G (D633), which are oxidized in viable cells to the mitochondrial stains rhodamine 123
and rhodamine 6G,
respectively
- Dihydroethidium (also known as hydroethidine; D1168, D11347, D23107), which forms the nucleic acid stain ethidium following oxidation
and has proven useful for detecting the viability of intracellular parasites
- Luminol (L8455), which is useful for chemiluminescence-based detection of oxidative events in cells rich in peroxidases, including granulocytes
and spermatozoa
These probes are all described in more detail in Generating and Detecting Reactive Oxygen Species—Section 18.2, which includes products for assaying oxidative activity in live cells and tissues.
 | Figure 15.2.10 An oxidative burst was detected by flow cytometry of cells labeled with 5-(and 6-)chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA, C6827). Jurkat cells were incubated with 100 nM CM-H2DCFDA. The cells were washed and resuspended in either phosphate-buffered saline (PBS, red) or PBS with 0.03% H2O2 (blue). The samples were analyzed on a flow cytometer equipped with a 488 nm argon-ion laser and a 525 ± 10 nm bandpass emission filter. |
Image-iT LIVE Green Reactive Oxygen Species Detection Kit
The Image-iT LIVE Green Reactive Oxygen Species Detection Kit (I36007) provides the key reagents for detecting reactive oxygen species (ROS) in live cells, including:
- Carboxy-H2DCFDA (5-(and 6-)carboxy-2',7'-dichlorodihydrofluorescein diacetate)
- Hoechst 33342
- tert-Butyl hydroperoxide (TBHP)
- Dimethylsulfoxide (DMSO)
- Detailed protocols for fluorescence microscopy assays (Image-iT LIVE Green Reactive Oxygen Species Detection Kit)
This assay is based on carboxy-H2DCFDA (5-(and 6-)carboxy-2',7'-dichlorodihydrofluorescein diacetate), a reliable fluorogenic marker for ROS in live cells.![]() In addition to carboxy-H2DCFDA, this kit provides the common inducer of ROS production tert-butyl hydroperoxide (TBHP) as a positive control
In addition to carboxy-H2DCFDA, this kit provides the common inducer of ROS production tert-butyl hydroperoxide (TBHP) as a positive control ![]() and the blue-fluorescent, cell-permeant nucleic acid stain Hoechst 33342. Oxidatively stressed and nonstressed cells can be reliable distinguished by fluorescence microscopy using this combination of dyes and the protocol provided (
and the blue-fluorescent, cell-permeant nucleic acid stain Hoechst 33342. Oxidatively stressed and nonstressed cells can be reliable distinguished by fluorescence microscopy using this combination of dyes and the protocol provided (![]() ).
).
RedoxSensor Red CC-1 Stain
RedoxSensor Red CC-1 stain (2,3,4,5,6-pentafluorotetramethyldihydrorosamine, R14060) passively enters live cells and is subsequently oxidized in the cytosol to a red-fluorescent product (excitation/emission maxima ~540/600 nm), which then accumulates in the mitochondria. Alternatively, this nonfluorescent probe may be transported to the lysosomes where it is oxidized. The differential distribution of the oxidized product between mitochondria and lysosomes appears to depend on the redox potential of the cytosol.![]() In proliferating cells, mitochondrial staining predominates; whereas in contact-inhibited cells, the staining is primarily lysosomal (
In proliferating cells, mitochondrial staining predominates; whereas in contact-inhibited cells, the staining is primarily lysosomal (![]() ).
).
Tetrazolium Salts
Tetrazolium salts are widely used for detecting redox potential of cells for viability, cytotoxicity and proliferation assays.![]() Following reduction, these water-soluble, colorless compounds form uncharged, brightly colored but nonfluorescent formazans. Several of the formazans precipitate out of solution and are useful for histochemical localization of the site of reduction or, after solubilization in organic solvent, for quantitation by standard spectrophotometric techniques.
Following reduction, these water-soluble, colorless compounds form uncharged, brightly colored but nonfluorescent formazans. Several of the formazans precipitate out of solution and are useful for histochemical localization of the site of reduction or, after solubilization in organic solvent, for quantitation by standard spectrophotometric techniques.
Reduction of MTT (M6494) remains the most common assay for tetrazolium salt–based viability testing.![]() The Vybrant MTT Cell Proliferation Assay Kit (V13154, Assays for Cell Enumeration, Cell Proliferation and Cell Cycle—Section 15.4) provides a simple method for determining cell number using standard microplate absorbance readers. MTT has also been used to measure adhesion of HL60 leukemia cells onto endothelial cells.
The Vybrant MTT Cell Proliferation Assay Kit (V13154, Assays for Cell Enumeration, Cell Proliferation and Cell Cycle—Section 15.4) provides a simple method for determining cell number using standard microplate absorbance readers. MTT has also been used to measure adhesion of HL60 leukemia cells onto endothelial cells.![]() In addition to dehydrogenases, MTT is reduced by glutathione S-transferase
In addition to dehydrogenases, MTT is reduced by glutathione S-transferase ![]() (GST). Therefore, MTT may not always be a reliable cell viability probe in cells treated with compounds that affect GST activity.
(GST). Therefore, MTT may not always be a reliable cell viability probe in cells treated with compounds that affect GST activity.
Unlike MTT's purple-colored formazan product, the extremely water-soluble, orange-colored formazan product of XTT (X6493) does not require solubilization prior to quantitation, thereby reducing the assay time in many viability assay protocols. Moreover, the sensitivity of the XTT reduction assay is reported to be similar to or better than that of the MTT reduction assay.![]() The XTT reduction assay is particularly useful for high-throughput screening of antiviral and antitumor agents and for assessing the effect of cytokines on cell proliferation.
The XTT reduction assay is particularly useful for high-throughput screening of antiviral and antitumor agents and for assessing the effect of cytokines on cell proliferation.![]() NBT (N6495) forms a deep blue–colored precipitate that is commonly used to indicate oxidative metabolism.
NBT (N6495) forms a deep blue–colored precipitate that is commonly used to indicate oxidative metabolism.![]()
A viable cell contains an ensemble of ion pumps and channels that maintain both intracellular ion concentrations and transmembrane potentials. Active maintenance of ion gradients ceases when the cell dies, and this loss of activity can be assessed using potentiometric dyes, acidotropic probes, Ca2+ indicators ![]() (Indicators for Ca2+, Mg2+, Zn2+ and Other Metal Ions—Chapter 19) and pH indicators
(Indicators for Ca2+, Mg2+, Zn2+ and Other Metal Ions—Chapter 19) and pH indicators ![]() (pH Indicators—Chapter 20).
(pH Indicators—Chapter 20).
Potentiometric Dyes
We offer a variety of dyes for detecting transmembrane potential gradients (Probes for Membrane Potential—Chapter 22), including several cationic probes that accumulate in the mitochondria of metabolically active cells (Probes for Mitochondria—Section 12.2). The mitochondrion-selective rhodamine 123 ![]() (R302, R22420) has been used to assess the viability of lymphocytes,
(R302, R22420) has been used to assess the viability of lymphocytes,![]() human fibroblasts,
human fibroblasts,![]() Simian virus–transformed human cells
Simian virus–transformed human cells ![]() and bacteria;
and bacteria;![]() however, rhodamine 123 is not taken up well by gram-negative bacteria.
however, rhodamine 123 is not taken up well by gram-negative bacteria.![]() Rhodamine 123 has also been used in combination with propidium iodide (P1304MP; P3566, P21493) for two-color flow cytometric viability assessment.
Rhodamine 123 has also been used in combination with propidium iodide (P1304MP; P3566, P21493) for two-color flow cytometric viability assessment.![]()
The methyl and ethyl esters of tetramethylrhodamine (T668, T669) accumulate in the mitochondria of healthy cells in an amount related to the membrane potential. The dyes are nontoxic and highly fluorescent and do not form aggregates or display binding-dependent changes in their fluorescence efficiency, permitting continuous monitoring of cell heath.![]()
Other potential-sensitive dyes that have proven useful in viability studies include several fast-response styryl dyes and slow-response oxonol and carbocyanine dyes. The fast-response styryl dyes such as di-4-ANEPPS (D1199, Fast-Response Probes—Section 22.2) give relatively large fluorescence response to potential changes. Di-4-ANEPPS was used for rapid measurement of toxicity in frog embryos.![]() The symmetrical bis-oxonol dyes
The symmetrical bis-oxonol dyes ![]() (B413, B438; Slow-Response Probes—Section 22.3) have been used for viability assessment by flow cytometry
(B413, B438; Slow-Response Probes—Section 22.3) have been used for viability assessment by flow cytometry ![]() and imaging. These slow-response dyes have also been employed to determine antibiotic susceptibility of bacteria by flow cytometry,
and imaging. These slow-response dyes have also been employed to determine antibiotic susceptibility of bacteria by flow cytometry,![]() and our BacLight Bacterial Membrane Potential Kit (B34950, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3) provides the carbocyanine dye DiOC2(3) along with the proton ionophore CCCP for detecting membrane potential in both gram-positive and gram-negative bacteria.
and our BacLight Bacterial Membrane Potential Kit (B34950, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3) provides the carbocyanine dye DiOC2(3) along with the proton ionophore CCCP for detecting membrane potential in both gram-positive and gram-negative bacteria.
The green-fluorescent cyanine dye JC-1 (5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolylcarbocyanine iodide, T3168, M34152) exists as a monomer at low concentrations or at low membrane potential; however, at higher concentrations (aqueous solutions above 0.1 µM) or higher potentials, JC-1 forms red-fluorescent "J-aggregates" (![]() ) that exhibit a broad excitation spectrum and an emission maximum at ~590 nm. JC-1 has been used to investigate apoptosis,
) that exhibit a broad excitation spectrum and an emission maximum at ~590 nm. JC-1 has been used to investigate apoptosis,![]() as well as mitochondrial poisoning, uncoupling and anoxia.
as well as mitochondrial poisoning, uncoupling and anoxia.![]() The ability to make ratiometric emission measurements with JC-1 makes this probe particularly useful for monitoring changes in cell health. We have discovered another carbocyanine dye, JC-9 (3,3'-dimethyl-β-naphthoxazolium iodide, D22421;
The ability to make ratiometric emission measurements with JC-1 makes this probe particularly useful for monitoring changes in cell health. We have discovered another carbocyanine dye, JC-9 (3,3'-dimethyl-β-naphthoxazolium iodide, D22421; ![]() ), with potential-dependent spectroscopic properties.
), with potential-dependent spectroscopic properties.
Acidotropic Stains
Membrane-bound proton pumps are used to maintain low pH within the cell's acidic organelles. Our complete selection of stains for lysosomes and other acidic organelles, including LysoTracker and LysoSensor probes, is described in Probes for Lysosomes, Peroxisomes and Yeast Vacuoles—Section 12.3.
The lysosomal stain neutral red (N3246), which was first used for viability measurements by Ehrlich in 1894, has been employed in numerous cytotoxicity, cell proliferation and adhesion assays.![]() Although usually used as a chromophoric probe, neutral red also fluoresces at ~640 nm in viable cells and has been detected using a fluorescence microplate reader.
Although usually used as a chromophoric probe, neutral red also fluoresces at ~640 nm in viable cells and has been detected using a fluorescence microplate reader.![]() Furthermore, the fluorescence of neutral red and BCECF AM (or SYTOX Green nucleic acid stain) can be measured simultaneously using a single excitation wavelength of 488 nm,
Furthermore, the fluorescence of neutral red and BCECF AM (or SYTOX Green nucleic acid stain) can be measured simultaneously using a single excitation wavelength of 488 nm,![]() suggesting that neutral red may be an effective probe for multicolor flow cytometric determination of cell viability. Our neutral red is highly purified to reduce contaminants that might interfere with these observations.
suggesting that neutral red may be an effective probe for multicolor flow cytometric determination of cell viability. Our neutral red is highly purified to reduce contaminants that might interfere with these observations.
Acridine orange (A1301, A3568) concentrates in acidic organelles in a pH-dependent manner. The metachromatic green or red fluorescence of acridine orange has been used to assess islet viability ![]() and bacterial spore viability
and bacterial spore viability ![]() and to monitor physiological activity in Escherichia coli.
and to monitor physiological activity in Escherichia coli.![]()
LysoTracker Green DND-26 (L7526, Probes for Lysosomes, Peroxisomes and Yeast Vacuoles—Section 12.3) was used in a fluorometric assay of cryopreserved sperm to demonstrate both acrosomal integrity and sperm viability.![]() Of several methods used, LysoTracker Green DND-26 staining was the quickest and easiest to use and gave excellent correlation with the SYBR 14 staining method used in our LIVE/DEAD Sperm Viability Kit (L7011, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3). When observed with a fluorescence microscope, sperm appeared to lose their green-fluorescent LysoTracker Green DND-26 staining and instead exhibit red-fluorescent propidium iodide staining within about 30 seconds after motility ceased.
Of several methods used, LysoTracker Green DND-26 staining was the quickest and easiest to use and gave excellent correlation with the SYBR 14 staining method used in our LIVE/DEAD Sperm Viability Kit (L7011, Viability and Cytotoxicity Assay Kits for Diverse Cell Types—Section 15.3). When observed with a fluorescence microscope, sperm appeared to lose their green-fluorescent LysoTracker Green DND-26 staining and instead exhibit red-fluorescent propidium iodide staining within about 30 seconds after motility ceased.![]()
Fluorescent Glucose Analogs
Measurements of glucose uptake can be used to assess viability in a variety of organisms. 2-NBD-deoxyglucose (2-NBDG, N13195) has been used to monitor glucose uptake in living pancreatic β-cells,![]() the yeast Candida albicans
the yeast Candida albicans![]() and the bacteria Escherichia coli.
and the bacteria Escherichia coli.![]() We also offer the fluorescent nonhydrolyzable glucose analog 6-NBD-deoxyglucose (6-NBDG, N23106). Using this probe, researchers have studied glucose uptake and transport in isolated cells
We also offer the fluorescent nonhydrolyzable glucose analog 6-NBD-deoxyglucose (6-NBDG, N23106). Using this probe, researchers have studied glucose uptake and transport in isolated cells ![]() and intact tissues.
and intact tissues.![]() Although sensitive to its environment, NBD fluorescence typically displays excitation/emission maxima of ~465/540 nm and can be visualized using optical filters designed for fluorescein.
Although sensitive to its environment, NBD fluorescence typically displays excitation/emission maxima of ~465/540 nm and can be visualized using optical filters designed for fluorescein.
Fluorescent Polymyxins
Polymyxin B is a cyclic cationic peptide antibiotic that binds to the lipopolysaccharide (LPS) component of the outer membrane of gram-negative bacteria and increases its permeability to lysozyme and hydrophobic compounds. Molecular Probes fluorescent BODIPY FL (P13235), Oregon Green 514 (P13236) and dansyl (P13238) derivatives of polymyxin B are available. Dansyl polymyxin B, which fluoresces weakly when free in solution, becomes highly fluorescent (excitation/emission ~340/485 nm) upon binding to intact cells or LPS.![]() The binding of dansyl polymyxin B to LPS can be displaced by a variety of polycationic antibiotics, as well as by Mg2+. Consequently, dansyl polymyxin B displacement experiments can be used to assess the binding of various compounds, such as antibiotics and macrophage cationic proteins, to LPS and intact bacterial cells.
The binding of dansyl polymyxin B to LPS can be displaced by a variety of polycationic antibiotics, as well as by Mg2+. Consequently, dansyl polymyxin B displacement experiments can be used to assess the binding of various compounds, such as antibiotics and macrophage cationic proteins, to LPS and intact bacterial cells.![]() Dansyl polymyxin has also been used to localize regions of high anionic lipid content in both sperm
Dansyl polymyxin has also been used to localize regions of high anionic lipid content in both sperm ![]() and aggregated human platelets,
and aggregated human platelets,![]() and to analyze the morphology of lipid bilayers in preparations of acetylcholine receptor clusters from rat myotubules.
and to analyze the morphology of lipid bilayers in preparations of acetylcholine receptor clusters from rat myotubules.![]() Green-fluorescent BODIPY FL and Oregon Green 514 polymyxin B provide additional fluorescent color options for these experiments. Our fluorescent LPS are described in Sphingolipids, Steroids, Lipopolysaccharides and Related Probes—Section 13.3 and Probes for Following Receptor Binding and Phagocytosis—Section 16.1.
Green-fluorescent BODIPY FL and Oregon Green 514 polymyxin B provide additional fluorescent color options for these experiments. Our fluorescent LPS are described in Sphingolipids, Steroids, Lipopolysaccharides and Related Probes—Section 13.3 and Probes for Following Receptor Binding and Phagocytosis—Section 16.1.
Fluorescent Penicillin Analogs
BOCILLIN FL penicillin and BOCILLIN 650/665 penicillin (B13233, B13234) are green- and infrared-fluorescent penicillin analogs, respectively, that bind selectively and with high affinity to penicillin-binding proteins present on the cytoplasmic membranes of eubacteria.![]() When electrophoresed under nonreducing conditions, the dye-labeled penicillin-binding proteins are easily visible in the gel with sensitivity in the low nanograms
When electrophoresed under nonreducing conditions, the dye-labeled penicillin-binding proteins are easily visible in the gel with sensitivity in the low nanograms ![]() (Figure 15.2.11). BOCILLIN FL penicillin, synthesized from penicillin V and the BODIPY FL dye (spectrally similar to fluorescein), has been used to determine the penicillin-binding protein profiles of Escherichia coli, Pseudomonas aeruginosa and Streptococcus pneumoniae, and these binding profiles are found to be similar to those reported by researchers using radioactively labeled penicillin V.
(Figure 15.2.11). BOCILLIN FL penicillin, synthesized from penicillin V and the BODIPY FL dye (spectrally similar to fluorescein), has been used to determine the penicillin-binding protein profiles of Escherichia coli, Pseudomonas aeruginosa and Streptococcus pneumoniae, and these binding profiles are found to be similar to those reported by researchers using radioactively labeled penicillin V.![]() Fluorescently labeled penicillin has also been used for direct labeling and rapid detection of whole E. coli and Bacillus licheniformis
Fluorescently labeled penicillin has also been used for direct labeling and rapid detection of whole E. coli and Bacillus licheniformis![]() and of Enterobacter pneumoniae.
and of Enterobacter pneumoniae.![]() The β-lactam sensor-transducer (BlaR), an integral membrane protein from Staphylococcus aureus, covalently and stoichiometrically reacts with β-lactam antibiotics, including BOCILLIN FL penicillin, by acylation of its active-site serine residue.
The β-lactam sensor-transducer (BlaR), an integral membrane protein from Staphylococcus aureus, covalently and stoichiometrically reacts with β-lactam antibiotics, including BOCILLIN FL penicillin, by acylation of its active-site serine residue.![]()
 | Figure 15.2.11 Detection of penicillin-binding proteins (PBPs) from Escherichia coli and Pseudomonas aeruginosa. |
BODIPY FL Vancomycin
BODIPY FL vancomycin (V34850), which contains a single BODIPY FL dye per vancomycin molecule, is a green-fluorescent analog of this important antibiotic, which is active against gram-positive bacteria, including enterococci. BODIPY FL vancomycin is useful for detecting vancomycin binding sites,![]() as well as vancomycin-resistant enterococci
as well as vancomycin-resistant enterococci ![]() (VRE, Probes for Cell Adhesion, Chemotaxis, Multidrug Resistance and Glutathione—Section 15.6).
(VRE, Probes for Cell Adhesion, Chemotaxis, Multidrug Resistance and Glutathione—Section 15.6).
Antimalarial Agent
The phosphonate antibiotic FR-31564 (fosmidomycin, F23103) is an effective antimalarial agents that function by blocking a mevalonate-independent methylerythritol phosphate (MEP) pathway of isoprene synthesis.![]() The antibiotic activity of fosmidomycin is potentiated by glucose 1-phosphate.
The antibiotic activity of fosmidomycin is potentiated by glucose 1-phosphate.![]() This antibiotic is also active against several gram-negative bacteria.
This antibiotic is also active against several gram-negative bacteria.![]()
| Cat # | MW | Storage | Soluble | Abs | EC | Em | Solvent | Notes |
|---|---|---|---|---|---|---|---|---|
| A1301 | 301.82 | L | H2O, EtOH | 500 | 53,000 | 526 | H2O/DNA | 1, 2 |
| A3568 | 301.82 | RR,L | H2O | 500 | 53,000 | 526 | H2O/DNA | 1, 2, 3 |
| B1150 | ~615 | F,D | DMSO | <300 | none | 4, 5 | ||
| B1170 | ~615 | F,D | DMSO | <300 | none | 4, 5 | ||
| B3051 | ~615 | F,D | DMSO | <300 | none | 3, 4, 5 | ||
| B13233 | 661.46 | F,D,L | H2O, DMSO | 504 | 68,000 | 511 | MeOH | |
| B13234 | 653.44 | F,D,L | DMSO | 646 | 78,000 | 659 | MeOH | |
| C195 | 460.40 | F,D | DMSO | <300 | none | 6 | ||
| C369 | 529.29 | F,D | DMSO | <300 | none | 7 | ||
| C400 | 531.30 | F,D | DMSO, EtOH | 290 | 5600 | none | MeCN | 8 |
| C1354 | 532.46 | F,D | DMSO | <300 | none | 9 | ||
| C1361 | 460.40 | F,D | DMSO | <300 | none | 6 | ||
| C1362 | 460.40 | F,D | DMSO | <300 | none | 6 | ||
| C1429 | 465.41 | F,D,L | DMSO | 322 | 13,000 | 437 | DMSO | 10 |
| C1430 | 994.87 | F,D | DMSO | <300 | none | 11 | ||
| C2925 | 464.86 | F,D | DMSO | <300 | none | 6 | ||
| C2938 | 675.43 | F,D,AA | DMSO | 291 | 5700 | none | MeOH | 8 |
| C3099 | 994.87 | F,D | DMSO | <300 | none | 3, 11 | ||
| C3100MP | 994.87 | F,D | DMSO | <300 | none | 11 | ||
| C6826 | 499.95 | F,D | DMSO | <350 | none | 12 | ||
| C6827 | 577.80 | F,D,AA | DMSO | 287 | 9100 | none | MeOH | 8 |
| C7025 | 464.86 | F,D | DMSO | <300 | none | 6 | ||
| C13196 | 560.52 | F,D | DMSO | <300 | none | 13 | ||
| C34851 | 789.55 | F,D,L | DMSO | 576 | 90,000 | 589 | DMSO | 14 |
| C34852 | 994.87 | F,D | DMSO | <300 | none | 11 | ||
| C34853 | 465.41 | F,D | DMSO | 322 | 13,000 | 437 | DMSO | 10 |
| D399 | 487.29 | F,D | DMSO, EtOH | 258 | 11,000 | none | MeOH | 8 |
| D632 | 346.38 | F,D,L,AA | DMF, DMSO | 289 | 7100 | none | MeOH | 15, 16 |
| D633 | 444.57 | F,D,L,AA | DMF, DMSO | 296 | 11,000 | none | MeOH | 15, 16 |
| D1168 | 315.42 | FF,L,AA | DMF, DMSO | 355 | 14,000 | see Notes | MeCN | 15, 17 |
| D11347 | 315.42 | FF,L,AA | DMF, DMSO | 355 | 14,000 | see Notes | MeCN | 15, 17 |
| D22421 | 532.38 | D,L | DMSO, DMF | 522 | 143,000 | 535 | CHCl3 | 18 |
| D23107 | 315.42 | FF,D,L,AA | DMSO | 355 | 14,000 | see Notes | MeCN | 17, 19 |
| D23805 | 1068.95 | F,D | DMSO | 285 | 5800 | none | MeCN | 20 |
| D23806 | 346.38 | F,D,L,AA | DMSO | 289 | 7100 | none | MeOH | 16, 19 |
| E1169 | 856.77 | F,D,L | DMSO | 528 | 7000 | 617 | H2O/DNA | 1, 21, 22 |
| E1374 | 420.31 | F,LL | DMF, EtOH | 462 | 5400 | 625 | pH 7 | 23 |
| E3599 | 1292.71 | F,D,L | DMSO | 535 | 8000 | 624 | H2O/DNA | 1, 3, 21, 22 |
| F1303 | 416.39 | F,D | DMSO | <300 | none | 6 | ||
| F7030 | 528.84 | F,D,L | DMSO | 508 | 71,000 | none | pH 7 | 3, 24 |
| F23103 | 205.08 | F,D,L | H2O | <300 | none | |||
| H7593 | 497.42 | L | DMSO | 518 | 3900 | 600 | H2O/DNA | 1, 25 |
| L8455 | 177.16 | D,L | DMF | 355 | 7500 | 411 | MeOH | 26 |
| M6494 | 414.32 | D,L | H2O, DMSO | 375 | 8300 | none | MeOH | 27, 28 |
| N3246 | 288.78 | D,L | H2O, EtOH | 541 | 39,000 | 640 | see Notes | 29 |
| N6495 | 817.65 | D,L | H2O, DMSO | 256 | 64,000 | none | MeOH | 27 |
| N13195 | 342.26 | F,L | H2O | 466 | 20,000 | 540 | MeOH | 30 |
| N23106 | 342.26 | F,L | DMSO | 475 | 25,000 | 552 | H2O | 30 |
| O6151 | 496.38 | F,D | DMSO | <300 | none | 31 | ||
| P1304MP | 668.40 | L | H2O, DMSO | 535 | 5400 | 617 | H2O/DNA | 1, 32 |
| P3566 | 668.40 | RR,L | H2O | 535 | 5400 | 617 | H2O/DNA | 1, 3, 32 |
| P21493 | 668.40 | L | H2O, DMSO | 535 | 5400 | 617 | H2O/DNA | 33 |
| R302 | 380.83 | F,D,L | MeOH, DMF | 507 | 101,000 | 529 | MeOH | |
| R12204 | 251.17 | L | H2O, MeOH | 604 | 60,000 | none | MeOH | 34 |
| R14060 | 434.41 | F,D,L,AA | DMSO | 239 | 52,000 | none | MeOH | 15, 35 |
| R22420 | 380.83 | F,D,L | MeOH, DMF | 507 | 101,000 | 529 | MeOH | 33 |
| S1129 | 518.43 | F,D | DMSO | <300 | none | 36 | ||
| S7020 | ~600 | F,D,L | DMSO | 504 | 67,000 | 523 | H2O/DNA | 1, 3, 37, 38 |
| S7575 | ~400 | F,D,L | DMSO | 488 | 74,000 | 509 | H2O/DNA | 1, 3, 37, 38, 39 |
| S11348 | ~400 | F,D,L | DMSO | 445 | 38,000 | 470 | H2O/DNA | 1, 3, 37, 38 |
| S11368 | ~500 | F,D,L | DMSO | 547 | 79,000 | 570 | H2O/DNA | 1, 3, 37, 38 |
| S32703 | ~800 | F,D,L | DMSO | 491 | 107,000 | 532 | H2O/RNA | 1, 3, 37, 38 |
| S32704 | ~350 | F,D,L | DMSO | 484 | 67,000 | 505 | H2O/DNA | 1, 3, 37, 38 |
| S34854 | ~400 | F,D,L | DMSO | 483 | 65,000 | 503 | H2O/DNA | 1, 3, 37, 38 |
| S34855 | ~400 | F,D,L | DMSO | 480 | 66,000 | 502 | H2O/DNA | 1, 3, 37, 38 |
| S34859 | ~450 | F,D,L | DMSO | 640 | 92,000 | 658 | H2O/DNA | 1, 3, 37, 38 |
| T668 | 500.93 | F,D,L | DMSO, MeOH | 549 | 115,000 | 573 | MeOH | |
| T669 | 514.96 | F,D,L | DMSO, EtOH | 549 | 109,000 | 574 | MeOH | |
| T3168 | 652.23 | D,L | DMSO, DMF | 514 | 195,000 | 529 | MeOH | 40 |
| V34850 | 1723.35 | F,D,L | H2O, DMSO | 504 | 68,000 | 511 | MeOH | |
| X6493 | 674.53 | F,D | H2O, DMSO | 286 | 15,000 | none | MeOH | 41 |
| ||||||||
For Research Use Only. Not for use in diagnostic procedures.